No products in the cart.
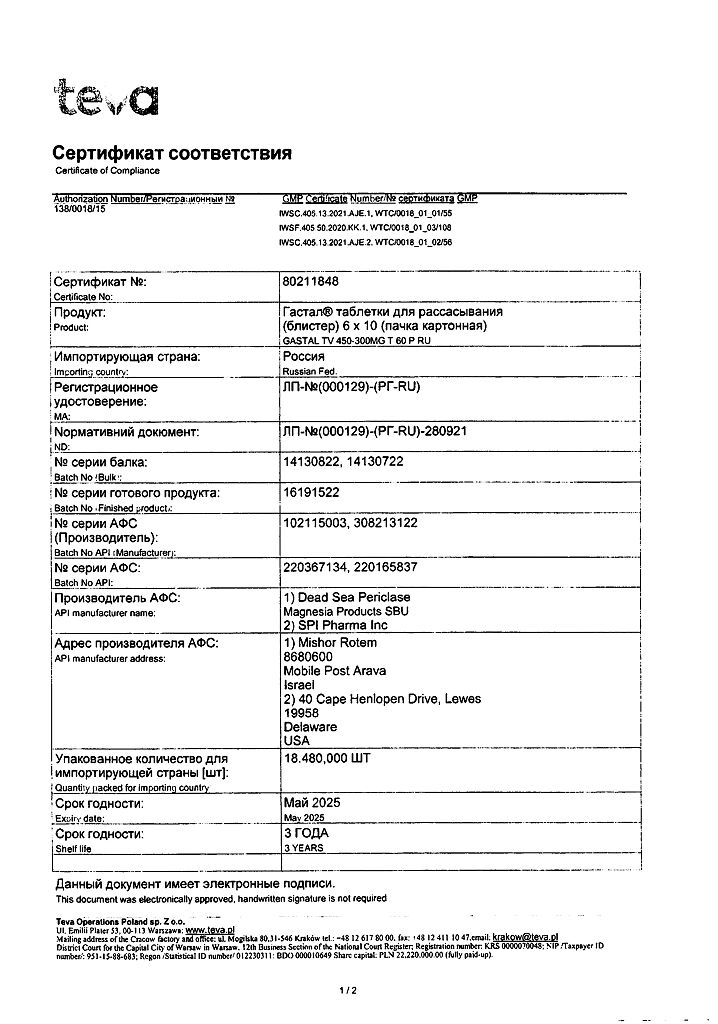
Gastal, tablets 60 pcs.
€16.62 €13.85
EAN: 3850114224017
SKU: 103614
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Pharmacotherapeutic group: antacid Drug ATX code: A02AD01 Pharmacological properties Pharmacodynamics Gastal® is a combined antacid drug that reduces increased acidity of gastric juice and has no stimulating effect on gastric juice secretion. Aluminum hydroxide-magnesium carbonate gel and magnesium hydroxide provide immediate (immediately after intake) and long-term (about 2 hours) neutralization of gastric hydrochloric acid, and maintenance of gastric acidity at physiological level (pH 3-5). One tablet of Gastal® neutralizes 21.5 mmol of hydrochloric acid. Gastal® inhibits the action of pepsin, lysolecithin and bile acids, eliminates dyspeptic symptoms. Strengthens protective and regenerative processes in gastric mucosa. Aluminum ions have cytoprotective effect due to increased secretion of mucin and sodium hydrocarbonate, activation of prostaglandin E2 and NO, accumulation of epidermal growth factor in place of mucous membrane injury, increased concentration of phospholipids in gastric walls. Pharmacokinetics Gastal® has no systemic effect in patients with normal renal function. After interaction with hydrochloric acid of gastric juice aluminum hydroxide reacts with phosphates and carbonates in alkaline intestinal environment and is excreted with feces as insoluble salts. Magnesium hydroxide reacts with hydrochloric acid in gastric juice to form magnesium chloride, which has osmotic properties and a mild laxative effect, which neutralizes the fixing effect of aluminum hydroxide in the small intestine. Magnesium ions are excreted with feces in the form of insoluble carbonate.
Indications
Indications
• Dyspeptic symptoms, such as: discomfort or pain in the epigastrium, heartburn, sour belching after errors in the diet, excessive consumption of alcohol, coffee, nicotine, etc.
• dyspepsia, such as: discomfort or pain in the epigastrium, heartburn, sour belching (and their prevention), resulting from the use of certain medications (non-steroidal anti-inflammatory drugs, glucocorticosteroids, etc.).
• conditions accompanied by increased acid formation: gastric ulcer, gastritis, reflux esophagitis; hiatal hernia.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antacid
ATX code: A02AD01
Pharmacological properties
Pharmacodynamics
The drug Gastal® is a combined antacid that reduces the increased acidity of gastric juice and does not have a stimulating effect on the secretion of gastric juice.
Aluminum hydroxide-magnesium carbonate gel and magnesium hydroxide provide immediate (immediately after administration) and long-term (about 2 hours) neutralization of hydrochloric acid in gastric juice, maintaining acidity in the stomach at a physiological level (pH 3-5). One tablet of Gastal® neutralizes 21.5 mmol of hydrochloric acid.
The drug Gastal® suppresses the effect of pepsin, lysolecithin and bile acids, eliminates dyspeptic symptoms. Strengthens protective and regenerative processes in the gastric mucosa. Aluminum ions have a cytoprotective effect by increasing the secretion of mucin and sodium bicarbonate, activating prostaglandin E2 and NO, accumulating epidermal growth factor at the site of mucosal damage, and increasing the concentration of phospholipids in the walls of the stomach.
Pharmacokinetics
The drug Gastal® does not have a systemic effect in patients with normal renal function. After interacting with the hydrochloric acid of gastric juice, aluminum hydroxide reacts with phosphates and carbonates in the alkaline environment of the intestine and is excreted in the feces in the form of insoluble salts. Magnesium hydroxide reacts with hydrochloric acid in gastric juice to form magnesium chloride, which has osmotic properties and a mild laxative effect that neutralizes the fixing effect of aluminum hydroxide in the small intestine. Magnesium ions are excreted in the feces in the form of insoluble carbonate.
Special instructions
Special instructions
The recommended dose and duration of treatment should not be exceeded when used in patients with impaired renal function.
In young children, use of magnesium hydroxide may cause hypermagnesemia, especially if they have renal failure or dehydration.
Impact on the ability to drive vehicles and machinery
The use of the drug Gastal® does not affect the ability to drive vehicles or control other mechanisms.
Active ingredient
Active ingredient
Aluminum hydroxide-magnesium carbonate, magnesium hydroxide
Composition
Composition
1 tablet contains: active ingredients: aluminum hydroxide-magnesium carbonate gel 450.00 mg, magnesium hydroxide 300.00 mg; excipients: mannitol (E421) 120.00 mg, sorbitol (E420) 50.00 mg, lactose monohydrate 30.00 mg, corn starch 75.80 mg, sodium cyclamate 7.00 mg, sodium saccharinate 0.20 mg, talc 28.00 mg, magnesium stearate 6.00 mg, mint flavor pepper 3.00 mg.
Pregnancy
Pregnancy
The drug Gastal® is not excreted in breast milk. When used during pregnancy and breastfeeding, it is necessary to evaluate the balance of benefit to the mother and risk to the fetus and infant. The drug should be used during pregnancy only if necessary and only on the advice of a doctor.
Contraindications
Contraindications
Hypersensitivity to aluminum, magnesium salts or other components of the drug; severe renal failure; Alzheimer’s disease; hypophosphatemia; lactose intolerance, lactase deficiency or glucose-galactose malabsorption; children under 6 years of age.
With caution
In case of chronic renal failure (CRF), pregnancy and during breastfeeding, adults and children over 12 years old weighing less than 50 kg, children aged 6 to 12 years, old age.
Side Effects
Side Effects
Adverse reactions are systematized in accordance with the World Health Organization (WHO) Classification: very common (?1/10); often (?1/100, <1/10); uncommon (?1/1000, <1/100); rare (?1/10000, <1/1000); very rare (< 1/10000); frequency unknown (cannot be determined from available data). Immune system disorders: very rarely - allergic reactions. Metabolic and nutritional disorders: very rarely - hypermagnesemia. Observed after long-term use of magnesium hydroxide in patients with renal failure Gastrointestinal disorders: frequency unknown - pain in the abdomen; rarely - nausea, diarrhea, constipation.
Interaction
Interaction
The drug Gastal®, when used simultaneously, enhances the activity of levodopa and nalidixic acid, reduces and slows down the absorption of quinolones, isoniazid, naproxen, iron preparations, indomethacin, aminazine, beta-blockers, diflunisal, H2-histamine receptor blockers, fat-soluble vitamins, indirect anticoagulants, barbiturates.
When interacting with metal ions included in antacids, tetracyclines form insoluble chelate complexes; as a result of this interaction, the absorption of tetracyclines is reduced by more than 90%. The simultaneous use of these drugs is not possible. If combined use is necessary, tetracycline should be taken at least 2 hours before taking the antacid.
In the presence of aluminum and magnesium hydroxides contained in the antacid, the absorption of ciprofloxacin and ofloxacin is reduced by 50-90%.
In the presence of antacids, the bioavailability of captopril is significantly reduced, and the combined use of antacids and metoprolol leads to a decrease in the concentration of metoprolol in the blood plasma.
Concomitant use of high doses of antacids can reduce the absorption of ranitidine by 10-33%.
The use of antacids does not affect the bioavailability of amoxicillin, cephalexin and the combination of amoxicillin and clavulanic acid, but can significantly reduce the absorption of doxycycline from the gastrointestinal tract.
An increase in urine pH during antacid therapy may increase the tubular reabsorption of basic (alkaline) drugs and reduce the reabsorption of acidic compounds. Antacids can reduce and slow down the absorption of salicylates, including acetylsalicylic acid, and also, by increasing urine pH, promote faster excretion of salicylates in the urine from the body, with a concomitant decrease in their concentration in the blood serum by 30-70%.
The absorption of cardiac glycosides, including digoxin and digitoxin, is not significantly reduced when used simultaneously with antacids.
M-anticholinergic blockers, by slowing down gastric motility, increase the duration of action of the drug Gastal®.
In order to prevent possible interaction of the drug Gastal® with other drugs, it is recommended to take it 1 hour before or 1 hour after their use.
Overdose
Overdose
Symptoms of acute overdose are not described.
With long-term use of high doses of drugs containing aluminum and magnesium, the development of hypophosphatemia, hypocalcemia, hypercalciuria, osteomalacia, osteoporosis, hypermagnesemia, hyperaluminemia, encephalopathy, nephrocalcinosis and impaired renal function is possible. It is possible to develop more pronounced adverse reactions from the gastrointestinal tract (gastrointestinal tract) (constipation, diarrhea), in patients with renal failure – thirst, decreased blood pressure, hyporeflexia.
Treatment: symptomatic therapy.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25? C.
Keep out of the reach of children!
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Teva Operations Poland Sp. z o.o., Poland
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ? Keep out of reach of children! |
| Manufacturer | Teva Operations Poland Sp. z o.o., Poland |
| Medication form | lozenges |
| Brand | Teva Operations Poland Sp. z o.o. |
Other forms…
Related products
Buy Gastal, tablets 60 pcs. with delivery to USA, UK, Europe and over 120 other countries.