No products in the cart.
Gairo, 500 mg 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
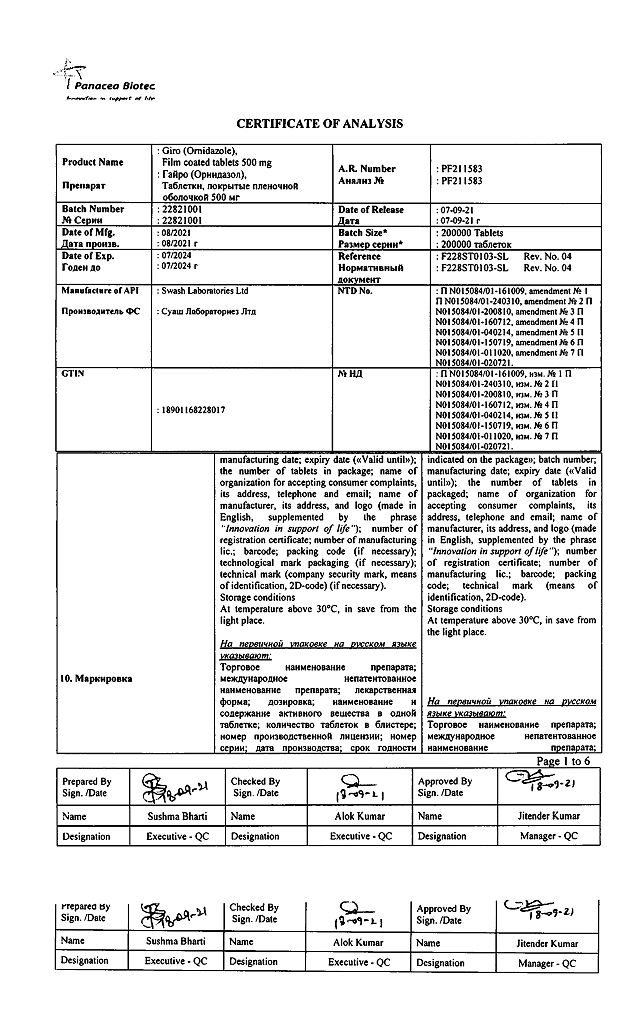
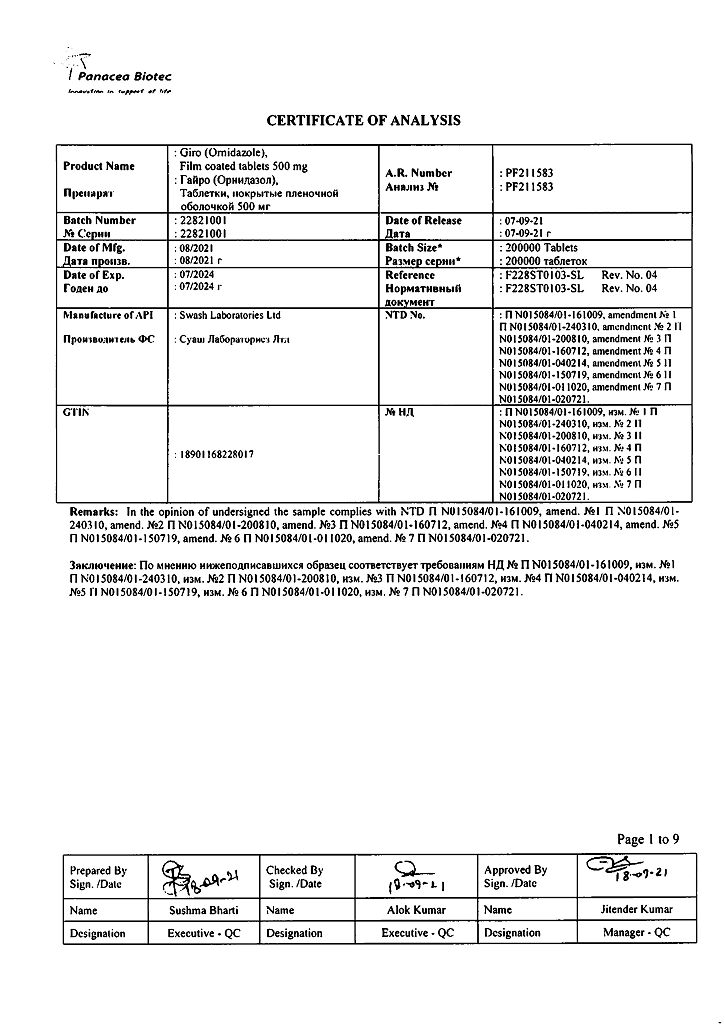
EAN: 8901168228010
SKU: 210071
Categories: Gynecology and Obstetrics, Medicine, Trichomoniasis and malaria
Description
Pharmacodynamics
Active against trichomonads, giardia, dysentery amoeba, some anaerobic microorganisms (bacteroides, fusobacteria, some strains of clostridia, anaerobic cocci).
Pharmacokinetics
Gastrointestinal absorption is high. Bioavailability – 90%. Tmax – 1-2 hours. Binding to plasma proteins – at least 15%. It penetrates through the BBB. T1/2 is about 13 hours. It is excreted as metabolites by kidneys (60-70%) and with feces (20-25%), about 5% of dose is excreted unchanged.
Indications
Indications
Trichomoniasis, amoebiasis, giardiasis, prevention of infections caused by anaerobic bacteria during operations on the colon and gynecology.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Active against Trichomonas, Giardia, dysenteric amoeba, some anaerobic microorganisms (bacteroides, fusobacteria, some strains of clostridia, anaerobic cocci).
Pharmacokinetics
Absorption from the gastrointestinal tract is high. Bioavailability – 90%. Tmax – 1-2 hours. Plasma protein binding – at least 15%. Penetrates through the BBB. T1/2 – about 13 hours. Excreted in the form of metabolites by the kidneys (60–70%) and with feces (20–25%), about 5% of the dose is excreted unchanged.
Special instructions
Special instructions
When treating trichomoniasis, both partners should be treated simultaneously.
Active ingredient
Active ingredient
Ornidazole
Composition
Composition
Film-coated tablets 1 table.
ornidazole500 mg
excipients:
MCC;
lactose;
Aerosil-200;
povidone (K-30);
magnesium stearate;
purified talc;
purified water;
sodium lauryl sulfate;
croscarmellose sodium
shell:
hypromellose (HPMC E-15); polyethylene glycol 400; purified talc; quinoline yellow; brilliant green; titanium dioxide; isopropyl alcohol; dichloromethane; polyethylene glycol 6000; distilled water
Pregnancy
Pregnancy
Contraindicated in the first trimester of pregnancy. Breastfeeding should be stopped during treatment.
Contraindications
Contraindications
Hypersensitivity, central nervous system diseases, pregnancy (first trimester), breastfeeding period.
Side Effects
Side Effects
Headache, dizziness, impaired consciousness, tremor, muscle rigidity, incoordination, convulsions, sensory or mixed peripheral neuropathy, nausea, vomiting, diarrhea.
Interaction
Interaction
Enhances the effect of coumarin anticoagulants and prolongs the muscle relaxant effect of vecuronium bromide. Compatible with alcohol (does not inhibit acetaldehyde dehydrogenase).
Overdose
Overdose
Symptoms: epileptiform seizures, depression, peripheral neuritis.
Treatment: symptomatic therapy (diazepam for seizures).
Storage conditions
Storage conditions
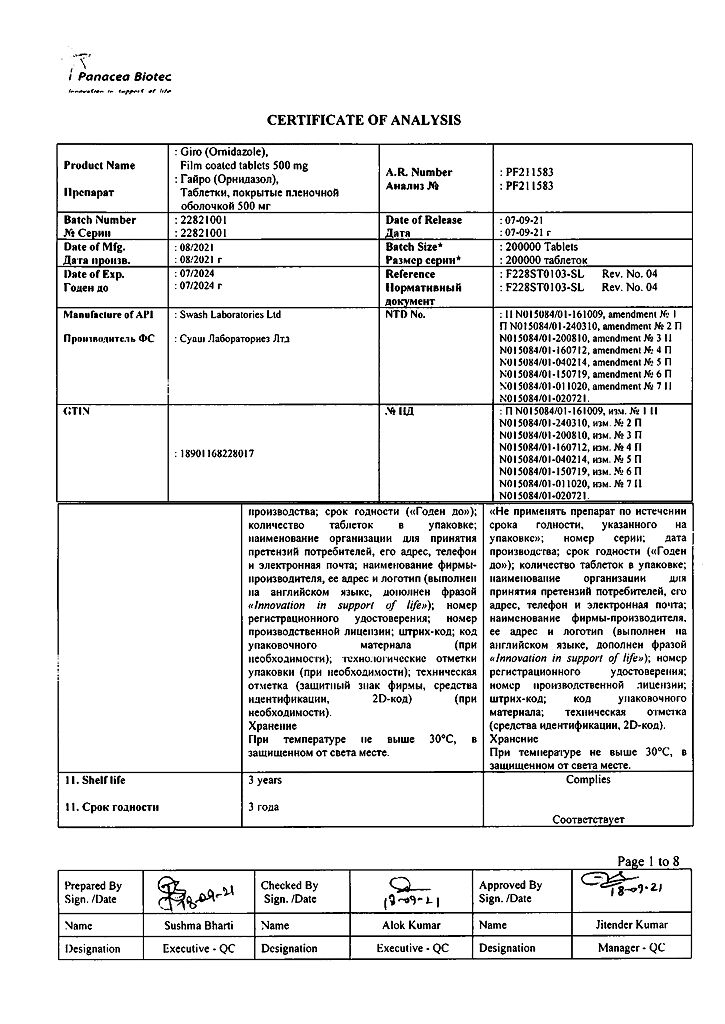
In a dry place, protected from light, at a temperature not exceeding 30 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Panacea Biotech, India
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 30 °C |
| Manufacturer | Panacea Biotec, India |
| Medication form | pills |
| Brand | Panacea Biotec |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Buy Gairo, 500 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.