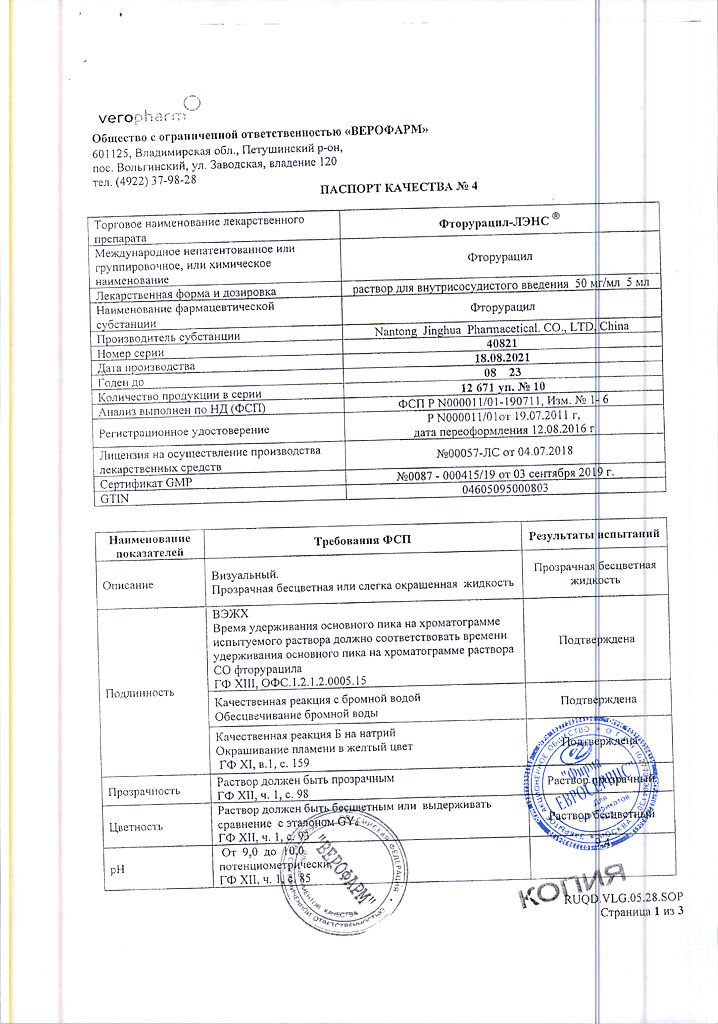
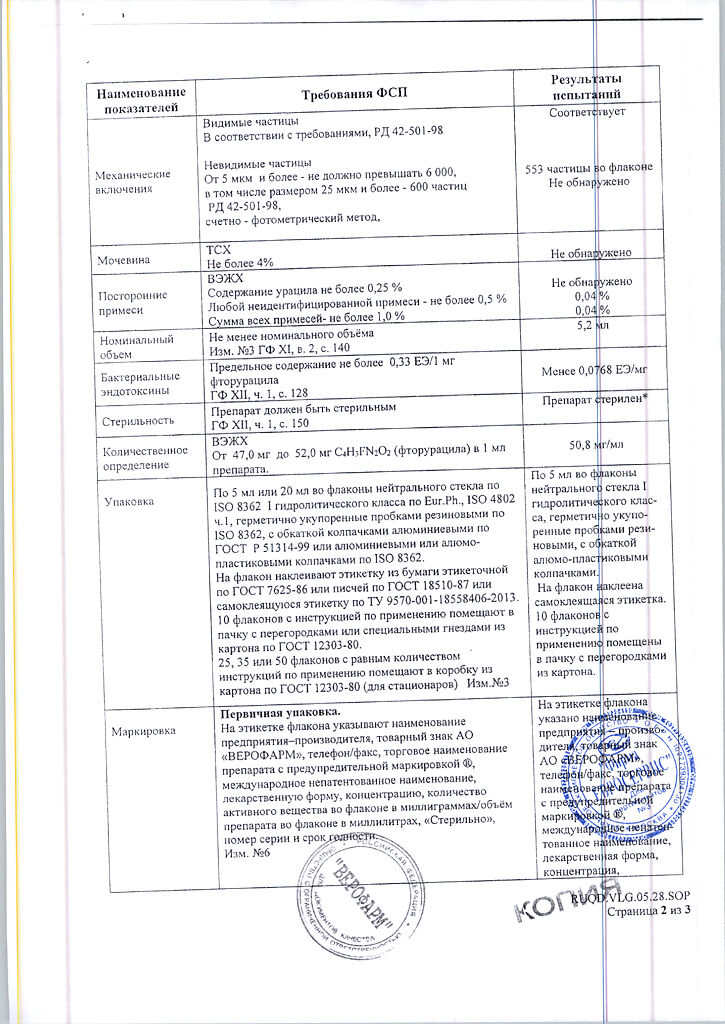
No products in the cart.
Description
Fluorouracil is an antimetabolite of uracil.
The mechanism of action is due to the conversion of the drug in the tissues into the active metabolite fluoruridinmonophosphate, which is a competitive inhibitor of the enzyme thymidylate synthetase, taking part in the synthesis of nucleic acids.
Fluorouracil disrupts DNA synthesis and causes formation of structurally imperfect RNA, inhibiting tumor cell division. Active metabolites are localized inside the cell
.
Indications
Indications
Colon and rectal cancer, breast cancer, esophageal cancer, stomach cancer, pancreatic cancer, primary liver cancer, ovarian cancer, cervical cancer, bladder cancer, malignant tumors of the head and neck, prostate cancer, adrenal cancer, penile cancer, carcinoid.
Pharmacological effect
Pharmacological effect
Fluorouracil is an antimetabolite of uracil.
The mechanism of action is due to the transformation of the drug in tissues into the active metabolite fluorouridine monophosphate, which is a competitive inhibitor of the enzyme thymidylate synthetase, which takes part in the synthesis of nucleic acids.
Fluorouracil disrupts DNA synthesis and causes the formation of structurally imperfect RNA, inhibiting the division of tumor cells. Active metabolites are localized inside the cell
Special instructions
Special instructions
Fluorouracil-LENS® is a cytotoxic drug, so care must be taken when handling it.
If stomatitis or diarrhea occurs, treatment with the drug must be stopped until these symptoms disappear.
The initial dose should be reduced by 1/3 or 1/2 in the following cases: weight loss, postoperative period of at least 30 days after major surgery, insufficient bone marrow function, impaired liver or kidney function.
Caution should be exercised when prescribing to patients who have previously been exposed to high doses of radiation to the pelvic area or who have received alkylating drugs.
During the treatment period, it is necessary to monitor the total number of leukocytes, the absolute number of neutrophils, platelets, determine hematocrit, hemoglobin, liver test activity and bilirubin level, and examine the patient’s oral cavity to identify signs of stomatitis.
Men and women of childbearing age should use reliable methods of contraception during treatment with Fluorouracil-LENS® and for at least 3 months after.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
Side effects caused by taking fluorouracil can negatively affect the ability to drive a vehicle and perform work that requires a high speed of psychomotor reactions.
Active ingredient
Active ingredient
Fluorouracil
Composition
Composition
1 ml of solution contains:
Active substance:
fluorouracil 50 mg
Excipients:
sodium hydroxide – 15.37 mg,
water for injection – up to 1 ml.
Pregnancy
Pregnancy
The drug is contraindicated during pregnancy and breastfeeding.
Contraindications
Contraindications
Hypersensitivity to fluorouracil and/or any other component of the drug;
pregnancy and breastfeeding;
severe leukopenia, neutropenia, thrombocytopenia;
stomatitis;
ulceration of the gastrointestinal mucosa, pseudomebranous enterocolitis.
With caution: use in case of renal and/or liver failure, acute infectious diseases of a viral, fungal or bacterial nature (including tuberculosis, chickenpox, herpes zoster), infiltration of the bone marrow by tumor cells, previous radiation therapy or chemotherapy.
Side Effects
Side Effects
From the hematopoietic organs: leukopenia, neutropenia, rarely – thrombocytopenia, anemia. The most significant drop in the number of leukocytes is usually observed from days 9 to 14 (up to day 25), platelets – from days 7 to 17 of treatment.
From the digestive system: loss of appetite, nausea, vomiting, inflammation and/or ulceration of the mucous membranes of the gastrointestinal tract (including stomatitis), diarrhea, bleeding from the gastrointestinal tract, heartburn and changes in taste, impaired liver function.
From the cardiovascular system: very rarely – pain in the heart, arrhythmias, ischemia, myocardial infarction, angina pectoris, heart failure.
From the nervous system: rarely – cerebellar ataxia, sensory disturbances, disorientation, confusion, euphoria, nystagmus, retrobulbar neuritis, headache.
From the senses: irritation of the mucous membrane of the eyes, excessive lacrimation due to ductal stenosis (10%-25%), photophobia, cataracts, cortical blindness (at high doses), visual impairment.
From the reproductive system: reversible inhibition of the function of the gonads, leading to amenorrhea or azoospermia.
From the skin and skin appendages: alopecia (rare), skin hyperpigmentation, dry and cracked skin, telangiectasia, palmoplantar erythrodysesthesia syndrome (tingling sensation in the hands and feet followed by pain, hyperemia and swelling), changes and convergence of the nail plates (rare), photosensitivity.
Allergic reactions: skin rash, dermatitis, urticaria, hyperemia of the skin of the palms and soles, bronchospasm, anaphylaxis (rare).
Other: fever (rare), thrombophlebitis: at the injection site, nosebleeds, cough, shortness of breath, hyperuricemia, weakness, development of secondary infections.
Interaction
Interaction
Calcium folinate enhances the therapeutic and toxic effects of fluorouracil. When used in combination with other cytostatics and interferon-alpha, an increase in both the antitumor effect and toxicity of fluorouracil may also be observed. With long-term combined use with mitomycin C, the appearance of hemolytic uremic syndrome was observed.
When taken concomitantly with the drug sorivudine, severe leukopenia was observed, in some cases leading to death.
Fluorouracil should not be used after or in combination with aminophenazone, phenylbutazone and sulfonamide therapy.
Chlordiazopoxide, disulfiram, griseofulvin and isoniazid may enhance the activity of 5 fluorouracil. Fluorouracil may reduce the immunological response to vaccination. When administered simultaneously with a live vaccine, severe antigenic reactions may develop.
Overdose
Overdose
Symptoms: nausea, vomiting, diarrhea, ulcerative stomatitis and gastric bleeding, suppression of bone marrow function (thrombocytopenia, leukopenia and agranulocytosis).
Treatment: symptomatic therapy. A specific antidote to fluorouracil is not known. In case of overdose, patients’ hematopoietic function should be monitored for at least 4 weeks.
Functional features
Functional features
After intravenous administration, the drug is quickly biotransformed and distributed
in tumor tissues, intestinal mucosa, bone marrow, liver and others
tissues. Easily penetrates the blood-brain barrier, entering the spinal cord
brain fluid and tissue. Metabolized mainly in the liver with
formation of inactive metabolites. The half-life of fluorouracil depends on
of the administered dose and is 8-22 minutes. About 20% of the drug is excreted by the kidneys in
unchanged for 6 hours (90% of this amount is excreted within 1
hours) and 60-80% – through the respiratory tract in the form of CO2, a small amount
excreted in bile.
Storage conditions
Storage conditions
At 15–25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Veropharm LLC, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At 15-25 °C |
| Manufacturer | Veropharm AO, Russia |
| Medication form | solution |
| Brand | Veropharm AO |
Related products
Buy Fluorouracil-LENS, 50 mg/ml 5 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.