No products in the cart.
Fluomizin, vaginal tablets 10 mg 6 pcs
€20.37 €16.97
Description
Fluomizin contains the active substance dequalinium chloride, which is a quaternary ammonium compound with a wide spectrum of antimicrobial activity. Dequalinia chloride is active against most Gram-positive bacteria Streptococcus spp. including β-haemolytic streptococci of groups A and B, Staphylococcus aureus, Listeria spp, anaerobes Peptostreptococcus (group D), Candida fungi (Candida tropicalis, Candida albicans, Candida glabrata), Gram-negative bacteria Gardnerella vaginalis, Escherichia coli, Serratia spp., Klebsiella spp., Pseudomonas spp., Proteus spp. and protozoa (Trichomonas vaginalis).
Pharmacokinetics
. When administered intravaginally, extremely small amounts of dequalinium chloride are absorbed through the vaginal mucosa into the systemic bloodstream, metabolized to a 2,2-dicarboxylic acid derivative and excreted in unconjugated form through the intestine.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
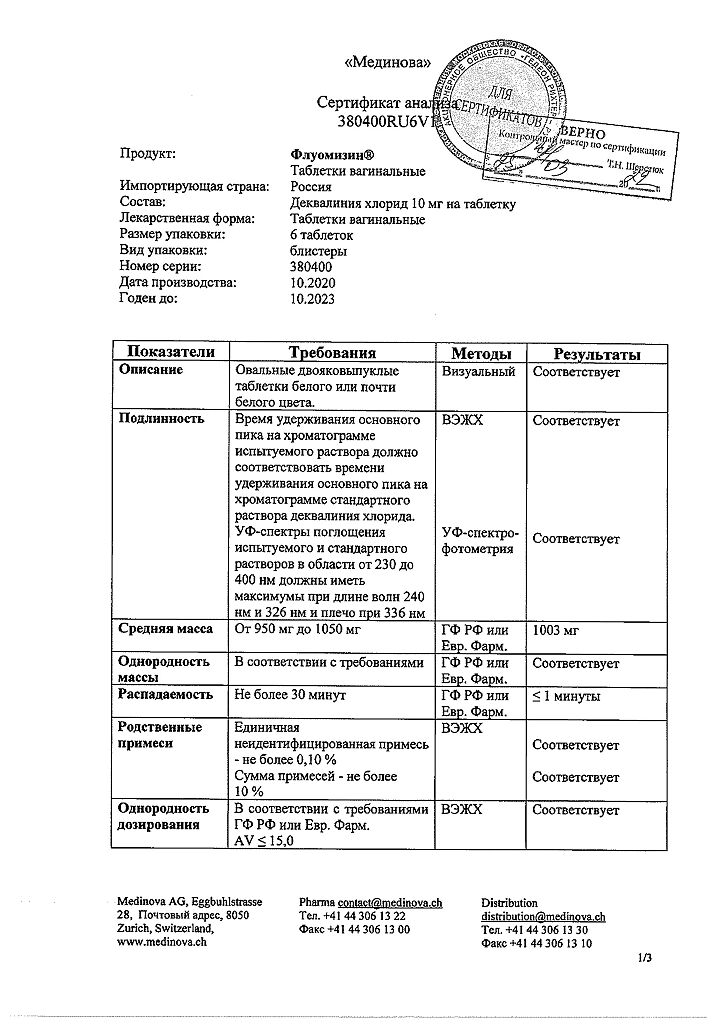
1 vaginal tablet contains:
the active ingredient:
dequalinium chloride 10 mg,
excipients:
Lactose monohydrate;
MCC;
Magnesium stearate
How to take, the dosage
How to take, the dosage
Intravaginally.
The recommended dosing regimen is 1 vaginal tablet/day. The vaginal tablet is inserted deep into the vagina in the evening before going to bed while lying on the back with the legs slightly bent.
The treatment should be stopped during menstruation and the previous course should be continued after it ends.
A full course of treatment (6 days) is necessary to avoid a relapse.
Interaction
Interaction
Fluomizin is incompatible with soaps and other anionic surfactants.
Special Instructions
Special Instructions
Fluomizin contains excipients that sometimes do not completely dissolve in the vagina. Therefore, the remains of the vaginal tablet can be found on the underwear. This does not affect the effectiveness of the drug Fluomizin.
In rare cases, if the vagina is excessively dry, there is a possibility that the pill will remain undissolved. To prevent this, the vaginal pill should be moistened with water (for 1 s under running water) before insertion.
With treatment, it is recommended that pads and underwear be changed more often. If clinical signs of infection persist after treatment has ended, repeat microbiological testing to identify the pathogen and confirm the diagnosis.
At the time of treatment with the drug, it is recommended to refrain from sexual intercourse. To prevent urogenital reinfection, simultaneous treatment of sexual partners is necessary.
Impact on the ability to drive a car or perform work requiring increased speed of physical and mental reactions. The drug does not affect the performance of potentially hazardous activities requiring special attention and quick psychomotor reactions (driving, etc.).
Contraindications
Contraindications
Side effects
Side effects
In extremely rare cases local irritation reactions (erosions), itching, burning or redness of the vaginal mucosa may occur. However, these adverse reactions may also be associated with symptoms of vaginal infection.
Extremely rare – fever, allergic reactions.
Overdose
Overdose
There are no data on overdose. An overdose is unlikely with intravaginal use.
Pregnancy use
Pregnancy use
The drug is approved for use throughout pregnancy and lactation.
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C. |
| Manufacturer | Medinova AG, Switzerland |
| Medication form | vaginal pills |
| Brand | Medinova AG |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Buy Fluomizin, vaginal tablets 10 mg 6 pcs with delivery to USA, UK, Europe and over 120 other countries.