No products in the cart.
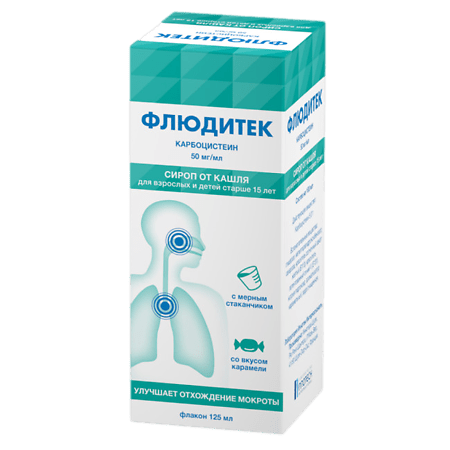
Fluditek, syrup 5% 125 ml
€14.83 €12.36
Description
The mucolytic and expectorant effect of Fluditek is due to the activation of sialic transferase – an enzyme of the bocalytic cells of the bronchial mucosa. It normalizes the quantitative ratio of acidic and neutral sialomucin of bronchial secretion, restores viscosity and elasticity of mucus.
Promotes regeneration of the mucous membrane, normalizes its structure, activates the ciliated epithelium. Restores the secretion of immunologically active IgA (specific protection) and the number of sulfhydryl groups of mucus components (nonspecific protection), improves mucociliary clearance.
Restores the secretion of immunologically active IgA (specific protection) and the number of sulfhydryl groups of mucus components (non-specific protection).
Pharmacokinetics
After oral administration Cmax in blood serum and in respiratory mucosa is reached after 2-3 hours and persists in mucosa for 8 hours. It is excreted mainly by the kidneys, partially – unchanged, partially – as metabolites.
Indications
Indications
Acute and chronic bronchopulmonary diseases, accompanied by the formation of viscous, difficult to separate sputum (tracheitis, bronchitis, tracheobronchitis, bronchial asthma, bronchiectasis).
Acute and chronic inflammatory diseases of the middle ear, nose and paranasal sinuses, accompanied by the formation of viscous, difficult-to-discharge mucus (rhinitis, otitis media, sinusitis).
Preparation for bronchoscopy and/or bronchography.
Pharmacological effect
Pharmacological effect
The mucolytic and expectorant effect of Fluditec is due to the activation of sialic transferase, an enzyme of goblet cells of the bronchial mucosa. Normalizes the quantitative ratio of acidic and neutral sialomucins in bronchial secretions, restores the viscosity and elasticity of mucus.
Promotes the regeneration of the mucous membrane, normalizes its structure, and activates the activity of the ciliated epithelium. Restores the secretion of immunologically active IgA (specific protection) and the amount of sulfhydryl groups of mucus components (nonspecific protection), improves mucociliary clearance.
Restores the secretion of immunologically active IgA (specific protection) and the amount of sulfhydryl groups of mucus components (non-specific protection).
Pharmacokinetics
After oral administration, Cmax in the blood serum and in the mucous membrane of the respiratory tract is reached within 2–3 hours and remains in the mucous membrane for 8 hours. It is excreted mainly by the kidneys, partially unchanged, partially in the form of metabolites.
Special instructions
Special instructions
Patients with diabetes or on a low-carbohydrate diet should take into account that 1 tablespoon of 5% syrup contains 5.25 g of sucrose. The presence of sucrose can cause flatulence and dyspepsia in young children.
When on a salt-free or low-salt diet, the sodium content of the drug should be taken into account.
Fluditec in the form of syrup 5% is intended only for adults and children over 15 years of age.
Active ingredient
Active ingredient
Carbocysteine
Composition
Composition
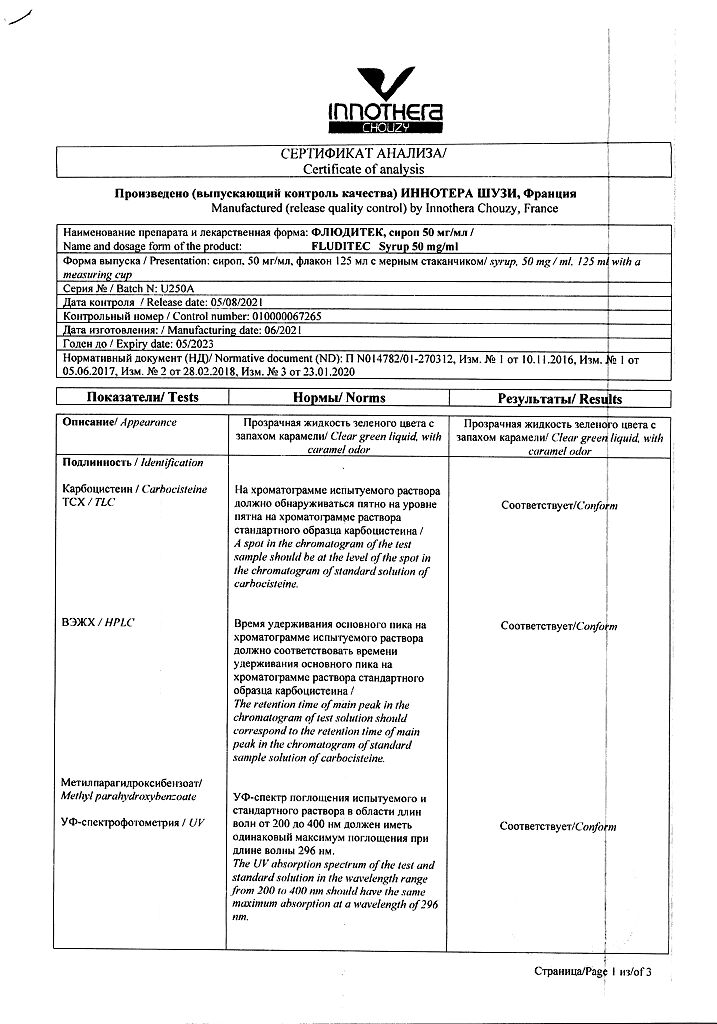
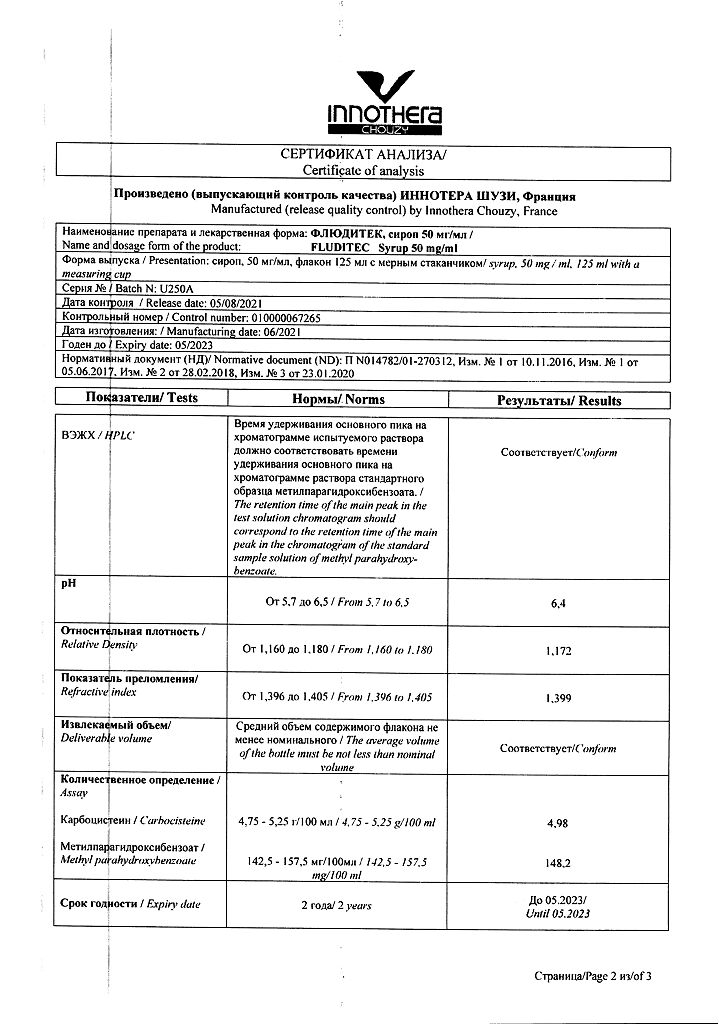
100 ml of syrup contains:
active ingredient:
carbocisteine 5 g
excipients:
sucrose,
caramel flavoring,
glycerin,
sodium hydroxide,
yellow-orange dye S,
purified water,
proprietary blue dye
preservative:
methyl parahydroxybenzoate
Pregnancy
Pregnancy
The use of Fluditec in the first trimester of pregnancy is contraindicated.
Fluditec should be prescribed with caution in the second and third trimesters of pregnancy and during breastfeeding.
Use in children
Contraindication: children aged 15 years.
Contraindications
Contraindications
hypersensitivity to carbocisteine or other components of the Fluditec drug;
peptic ulcer of the stomach and duodenum (in the acute stage);
chronic glomerulonephritis (in the acute stage), cystitis;
children under 15 years of age;
pregnancy (first trimester).
With caution: chronic glomerulonephritis (history); peptic ulcer of the stomach and duodenum (history); pregnancy (II–III trimester); breastfeeding period.
Side Effects
Side Effects
Nausea, vomiting, diarrhea, epigastric pain, flatulence, gastrointestinal bleeding, dizziness, weakness, malaise, in isolated cases – allergic reactions (itching, urticaria, exanthema, angioedema).
If any of the side effects indicated in the description worsen, or the patient notices any other side effects not listed in the description, the doctor should be informed.
Interaction
Interaction
With simultaneous use, Fluditec increases the effectiveness of glucocorticosteroid (mutually) and antibacterial therapy for inflammatory diseases of the upper and lower respiratory tract.
With simultaneous use, Fluditec enhances the bronchodilator effect of theophylline.
The activity of carbocisteine is weakened by antitussives and atropine-like drugs.
Overdose
Overdose
Symptoms: gastralgia, nausea, diarrhea.
Treatment: symptomatic therapy.
Storage conditions
Storage conditions
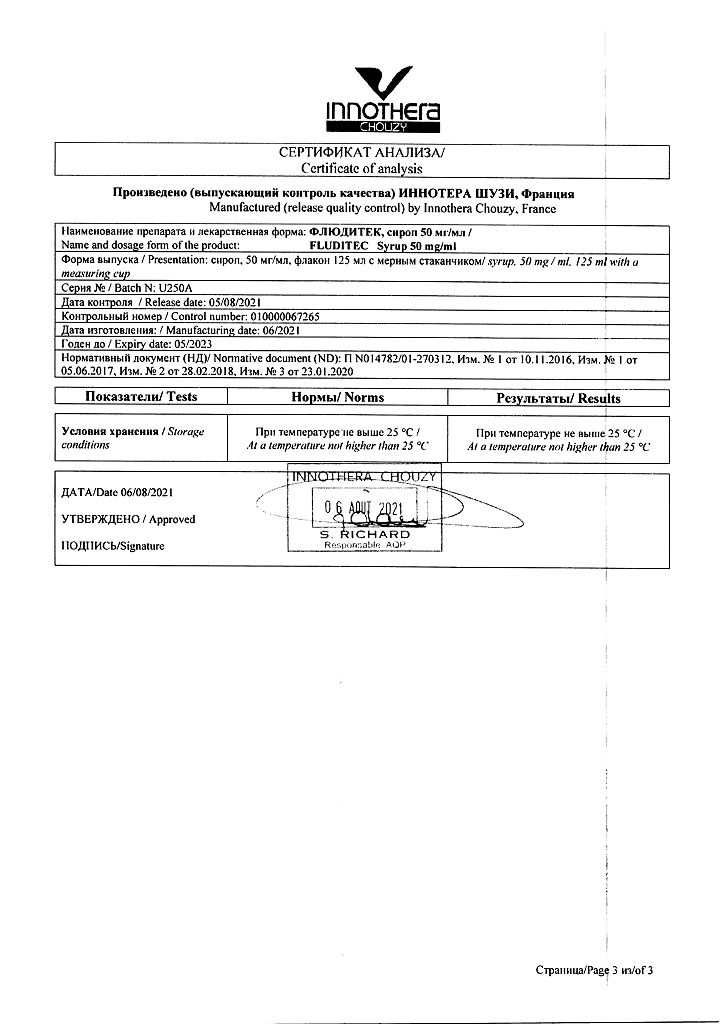
At a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Uniter Liquid Manufacturing, France
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Innotech International, France |
| Medication form | syrup |
| Brand | Innotech International |
Related products
Buy Fluditek, syrup 5% 125 ml with delivery to USA, UK, Europe and over 120 other countries.