No products in the cart.
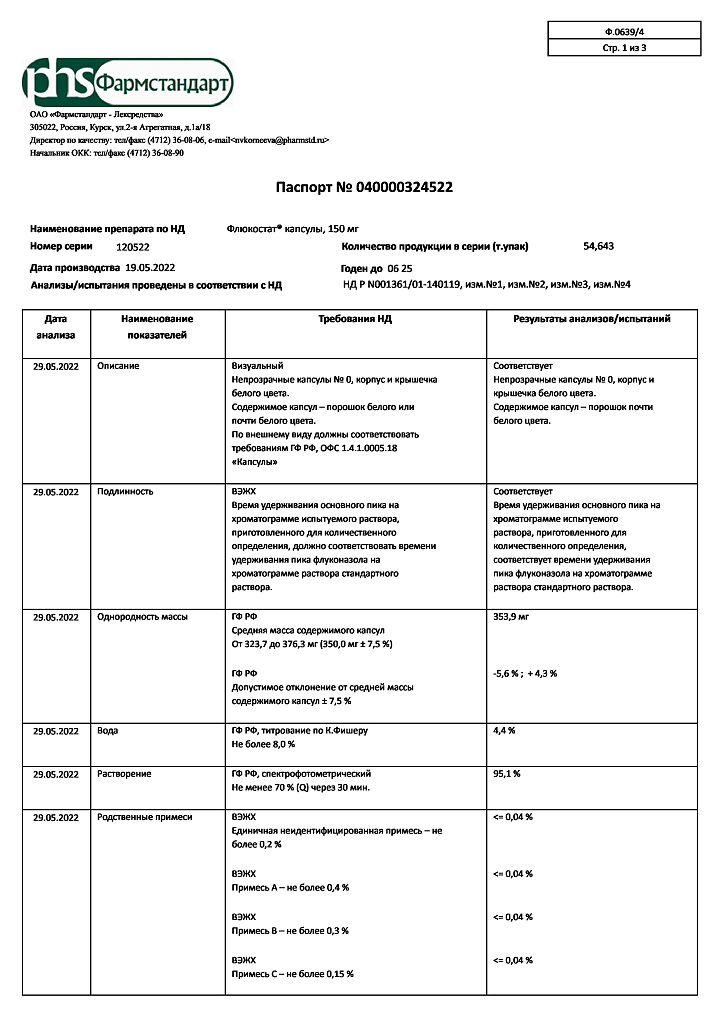
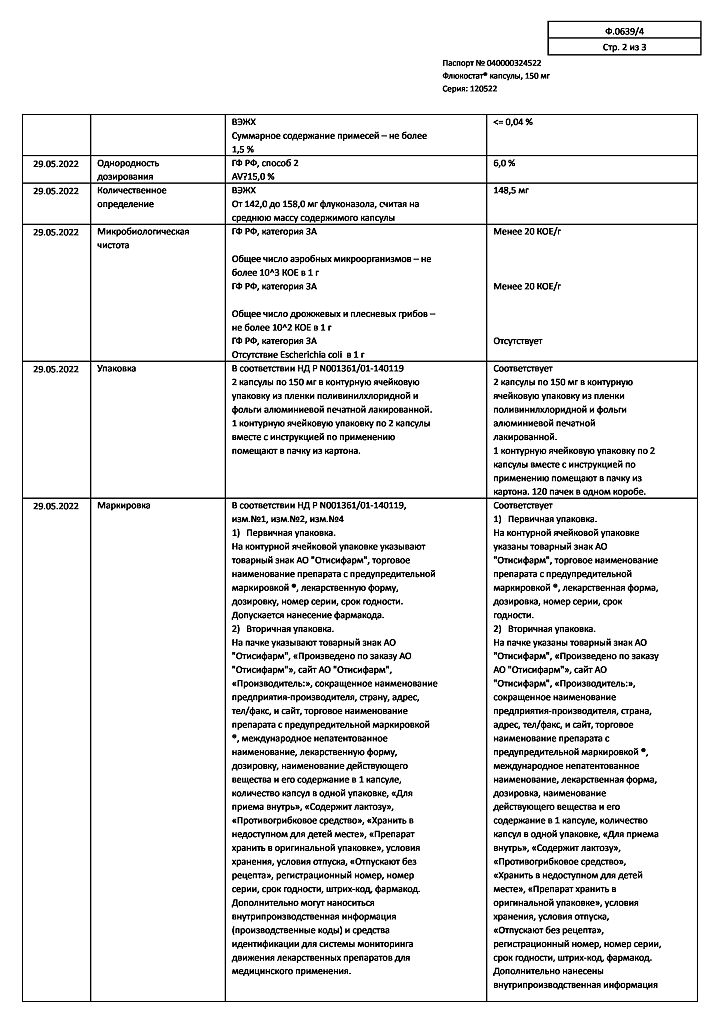
Flucostat, 150 mg capsules 2 pcs
€8.00 €7.18
Description
Pharmacodynamics:
Fluconazole, a member of the triazole antifungal class, is a selective inhibitor of sterol synthesis in the fungal cell.
Fluconazole has demonstrated activity in-vitro and in clinical studies against most of the following microorganisms: Candidaalbicans, Candidaglabrata (many strains are moderately sensitive), Candidaparapsilosis, Candidatropicalis, Cryptococcusneoformans.
The activity of fluconazole against the following microorganisms has been shown, but its clinical significance is not known: Candidadubliniensis, Candidaguilliermondii, Candidakefyr, Candidalusitaniae.
In oral administration, fluconazole shows activity in various models of fungal infections in animals. It has demonstrated activity against opportunistic mycoses including those caused by Candidaspp. (including generalized candidiasis in immunocompromised animals), Cryptococcus neoformans (including intracranial infections), Microsporumspp. and Trychophytonspp.
Fluconazole has high specificity against cytochrome P450-dependent fungal enzymes. Fluconazole therapy at a dose of 50 mg/day for up to 28 days has no effect on plasma testosterone concentrations in men or steroid concentrations in women of childbearing age.
Fluconazole at a dose of 200 to 400 mg/day has no clinically significant effect on endogenous steroid levels and their response to adrenocorticotropic hormone (ACTH) stimulation in healthy male volunteers.
Mechanisms for the development of resistance to fluconazole
Resistance to fluconazole can develop in the following cases: a qualitative and quantitative change in the enzyme that targets fluconazole (lanosterol 14-α-demethylase), a decrease in access to the fluconazole target, or a combination of these mechanisms.
Point mutations in the ERG11 gene, which encodes the target enzyme, lead to target alteration and decreased affinity for azoles. Increased expression of the ERG11 gene leads to production of high concentrations of the target enzyme, which creates a need for increased fluconazole concentrations in the intracellular fluid to inhibit all enzyme molecules in the cell.
The second significant mechanism of resistance is the active excretion of fluconazole from the intracellular space through the activation of two types of transporters involved in the active excretion (efflux) of the drugs from the fungal cell.
These transporters include a major mediator encoded by the MDR (multidrug resistance) genes and an ATP-binding cassette transporter superfamily encoded by the CDR genes (Candida fungi resistance genes to azolovymantimycotics).
Hyperexpression of the MDR gene leads to resistance to fluconazole, while hyperexpression of CDR genes can lead to resistance to various azoles.
Resistance to Candidaglabrata is usually mediated by CDR gene overexpression, which leads to resistance to many azoles. For those strains in which the minimum inhibitory concentration (MIC) is defined as intermediate (16-32 µg/ml), maximum doses of fluconazole are recommended.
Candidacrosis should be considered resistant to fluconazole. The mechanism of resistance is associated with reduced sensitivity of the target enzyme to the inhibitory effects of fluconazole.
Pharmacokinetics:
Fluconazole pharmacokinetics are similar with intravenous and oral administration. After oral administration fluconazole is well absorbed, its plasma concentrations (and overall bioavailability) exceed 90% of those of intravenous administration.
Contemporaneous intake of food does not affect absorption of the drug taken orally. The plasma concentration is proportional to the dose and reaches a maximum (Cmax) in 0.5-1.5 hours after fluconazole is taken on an empty stomach, and the elimination half-life is about 30 hours.
The maximum concentration of fluconazole in saliva when taking the capsule is reached after 4 hours.
The volume of distribution is close to the total water content in the body. Binding to plasma proteins is low (11-12%).
Fluconazole penetrates well into all body fluids. Concentrations of the drug in saliva and sputum are similar to its levels in blood plasma.
In the stratum corneum, epidermis, dermis and sweat fluids, high concentrations are achieved that exceed serum levels.
Fluconazole accumulates in the stratum corneum. When taken at a dose of 50 mg once daily, fluconazole concentrations are 73 µg/g after 12 days and only 5.8 µg/g after 7 days of discontinuation of treatment. When used in a dose of 150 mg once a week, the concentration of fluconazole in the stratum corneum on day 7 is 23.4 µg/g, and 7 days after the second dose is 7.1 µg/g.
The concentration of fluconazole in the nails after 4 months of use at a dose of 150 mg once a week is 4.05 in healthy nails and 1.8 µg/g in affected nails; 6 months after completion of therapy, fluconazole is still detectable in the nails.
Fluconazole is mainly excreted by the kidneys; approximately 80% of the administered dose is excreted unchanged. Fluconazole clearance is proportional to creatinine clearance. No fluconazole metabolites were detected in peripheral blood.
The long plasma elimination half-life allows fluconazole to be taken once for vaginal candidiasis.
Pharmacokinetics in elderly patients
It was found that with a single oral dose of fluconazole 50 mg in elderly patients aged 65 years and older, some of whom were also taking diuretics, Cmax was reached 1.3 h after administration and was 1.54 µg/mL, the mean AUC (area under the concentration-time curve) was 76.4 ± 20.3 µg×h/mL, and the mean half-life was 46.2 h.
The values of these pharmacokinetic parameters are higher than in younger patients, which is probably due to the reduced renal function characteristic of older age. Concomitant administration of diuretics did not cause marked changes in AUC and Cmax.
Creatinine clearance (74 ml/min), the percentage of fluconazole excreted unchanged by the kidneys (0-24 h, 22%) and the renal clearance of fluconazole (0.124 ml/min/kg) are lower in elderly patients compared to younger patients.
Indications
Indications
Treatment
acute vaginal candidiasis, when local therapy is not applicable.
Pharmacological effect
Pharmacological effect
Pharmacodynamics:
Fluconazole, a member of the triazole antifungal class, is a selective inhibitor of sterol synthesis in fungal cells.
Fluconazole has demonstrated activity in vitro and in clinical studies against most of the following microorganisms: Candida albicans, Candidaglabrata (many strains are moderately sensitive), Candidaparapsilosis, Candida tropicalis, Cryptococcus neoformans.
Fluconazole has been shown to be active in vitro against the following microorganisms, but its clinical significance is unknown: Candidadubliniensis, Candidaguilliermondii, Candidakefyr, Candidalusitaniae.
When administered orally, fluconazole exhibits activity in various animal models of fungal infections. The drug has been demonstrated to be active against opportunistic mycoses, including those caused by Candidaspp. (including generalized candidiasis in immunosuppressed animals), Cryptococcus neoformans (including intracranial infections), Microsporum spp. and Trychophytonspp.
Fluconazole has high specificity for fungal enzymes dependent on cytochrome P450. Fluconazole therapy at a dose of 50 mg/day for up to 28 days does not affect plasma testosterone concentrations in men or steroid concentrations in women of childbearing age.
Fluconazole at a dose of 200 – 400 mg/day does not have a clinically significant effect on the levels of endogenous steroids and their response to adrenocorticotropic hormone (ACTH) stimulation in healthy male volunteers.
Mechanisms of development of resistance to fluconazole
Resistance to fluconazole can develop in the following cases: a qualitative and quantitative change in the enzyme that is the target of fluconazole (lanosteryl 14-α-demethylase), a decrease in access to the target of fluconazole, or a combination of these mechanisms.
Point mutations in the ERG11 gene, encoding the target enzyme, lead to a modification of the target and a decrease in affinity for azoles. An increase in the expression of the ERG11 gene leads to the production of high concentrations of the target enzyme, which creates the need to increase the concentration of fluconazole in the intracellular fluid to suppress all enzyme molecules in the cell.
The second significant mechanism of resistance is the active removal of fluconazole from the intracellular space through the activation of two types of transporters involved in the active removal (efflux) of drugs from the fungal cell.
These transporters include the master messenger, encoded by the MDR (multidrug resistance) genes, and the ATP-binding cassette transporter superfamily, encoded by the CDR genes (Candida azole antimycotic resistance genes).
Overexpression of the MDR gene leads to resistance to fluconazole, while overexpression of CDR genes can lead to resistance to various azoles.
Resistance to Candidaglabrata is usually mediated by overexpression of the CDR gene, leading to resistance to many azoles. For those strains in which the minimum inhibitory concentration (MIC) is determined to be intermediate (16-32 μg/ml), it is recommended to use the maximum dose of fluconazole.
Candidakrusei should be considered resistant to fluconazole. The mechanism of resistance is associated with reduced sensitivity of the target enzyme to the inhibitory effects of fluconazole.
Pharmacokinetics:
The pharmacokinetics of fluconazole are similar when administered intravenously and orally. After oral administration, fluconazole is well absorbed, its plasma concentration (and total bioavailability) exceeds 90% of those when administered intravenously.
Concomitant food intake does not affect the absorption of the drug taken orally. Plasma concentrations are proportional to the dose and reach a maximum (Cmax) 0.5-1.5 hours after taking fluconazole on an empty stomach, and the half-life is about 30 hours.
The maximum concentration of fluconazole in saliva when taking the capsule is reached after 4 hours.
The volume of distribution approaches the total water content of the body. Binding to blood plasma proteins is low (11-12%).
Fluconazole penetrates well into all biological fluids of the body. Concentrations of the drug in saliva and sputum are similar to its levels in blood plasma.
In the stratum corneum, epidermis, dermis and sweat fluid, high concentrations are reached that exceed serum concentrations.
Fluconazole accumulates in the stratum corneum. When taken at a dose of 50 mg once daily, the concentration of fluconazole after 12 days is 73 mcg/g, and 7 days after stopping treatment it is only 5.8 mcg/g. When used at a dose of 150 mg once a week, the concentration of fluconazole in the stratum corneum on the 7th day is 23.4 mcg/g, and 7 days after taking the second dose – 7.1 mcg/g.
The concentration of fluconazole in nails after 4 months of use at a dose of 150 mg once a week is 4.05 in healthy and 1.8 mcg/g in affected nails; 6 months after completion of therapy, fluconazole is still detected in the nails.
Fluconazole is excreted primarily by the kidneys; approximately 80% of the administered dose is excreted unchanged. Fluconazole clearance is proportional to creatinine clearance. No fluconazole metabolites were detected in peripheral blood.
The long half-life from blood plasma allows fluconazole to be taken once for vaginal candidiasis.
Pharmacokinetics in elderly patients
It was found that with a single dose of fluconazole 50 mg orally in elderly patients aged 65 years and older, some of whom were concomitantly taking diuretics, the Cmax was reached 1.3 hours after dosing and was 1.54 μg/ml, the mean AUC (area under the concentration-time curve) was 76.4 ± 20.3 μg × h/ml, and the mean elimination half-life was 46.2 hours
The values of these pharmacokinetic parameters are higher than in young patients, which is likely due to reduced renal function characteristic of old age. Concomitant use of diuretics did not cause a significant change in AUC and Cmax.
Creatinine clearance (74 ml/min), the percentage of fluconazole excreted unchanged by the kidneys (0-24 hours, 22%) and renal clearance of fluconazole (0.124 ml/min/kg) are lower in elderly patients compared to young patients.
Special instructions
Special instructions
When using fluconazole 150 mg for vaginal candidiasis, patients should be warned that improvement in symptoms is usually observed after 24 hours, but sometimes takes several days for complete resolution. If symptoms persist for several days, you should consult a doctor.
In rare cases, the use of fluconazole was accompanied by toxic changes in the liver, incl. with a fatal outcome, mainly in patients with serious concomitant diseases.
In the case of hepatotoxic effects associated with fluconazole, there was no obvious dependence on the total daily dose, duration of therapy, gender and age of the patient. The hepatotoxic effects of fluconazole were usually reversible; its signs disappeared after cessation of therapy.
Patients who experience abnormal liver function tests during drug treatment should be monitored for signs of more serious liver damage. If clinical signs or symptoms of liver damage appear that may be associated with the use of fluconazole, the drug should be discontinued.
As with other azoles, fluconazole can rarely cause anaphylactic reactions.
During treatment with fluconazole, patients have rarely developed exfoliative skin lesions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.
People with AIDS are more likely to develop severe skin reactions when taking many drugs. In cases where a rash develops in patients with a superficial fungal infection and is assessed as definitely related to fluconazole, the drug should be discontinued.
If a rash appears in patients with invasive/systemic fungal infections, they should be carefully monitored and fluconazole should be discontinued if bullous changes or erythema multiforme exudative lesions occur.
The simultaneous use of fluconazole in doses less than 400 mg/day and terfenadine should be carried out under close supervision (see section “Interaction with other drugs”).
Like other azoles, fluconazole may cause prolongation of the QT interval on the ECG. When using fluconazole, an increase in the QT interval and ventricular fibrillation or flutter were observed very rarely in patients with severe diseases with multiple risk factors, such as organic heart disease, electrolyte imbalances, and concomitant therapy that contributes to the development of such disorders. Therefore, fluconazole should be used with caution in such patients with potentially proarrhythmic conditions.
Patients with liver, heart and kidney diseases are advised to consult a doctor before using the drug.
Superinfections caused by strains of Candida other than Candida albicans, which are often intrinsically resistant to fluconazole (eg, Candidakrusei), have been reported. In such cases, alternative antifungal therapy may be required.
Impact on the ability to drive vehicles and machinery
Due to the possibility of dizziness and other side effects associated with taking the drug, during the treatment period patients are advised to refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration, speed of psychomotor and motor reactions.
Active ingredient
Active ingredient
Fluconazole
Composition
Composition
Active ingredient:
fluconazole 50 mg or 150 mg;
excipients: lactose (milk sugar) 49.40 mg or 147.40 mg, corn starch 16.40 mg or 49.00 mg, colloidal silicon dioxide (aerosil) 0.12 mg or 0.36 mg, magnesium stearate 0.96 mg or 2.88 mg, sodium lauryl sulfate 0.12 mg or 0.36 mg;
hard gelatin capsules:
(for a dosage of 50 mg) body: titanium dioxide (E 171) – 3.0000%, red iron oxide (E 172) – 0.0857%, gelatin – up to 100%; cap: titanium dioxide (E 171) – 2.0000%, red iron oxide (E 172) – 0.7286%, gelatin – up to 100%;
(for a dosage of 150 mg) body and cap: titanium dioxide (E 171) – 2.0000%, gelatin – up to 100%.
Pregnancy
Pregnancy
There have been no adequate and controlled studies of the use of fluconazole in pregnant women.
Currently, there is no evidence of the effect of low doses of fluconazole (150 mg once for the treatment of vulvovaginal candidiasis) on the increase in the incidence of adverse pregnancy outcomes, as well as the relationship with the occurrence of any specific malformations in the child.
When using high doses (400-800 mg/day) of fluconazole, several cases of multiple congenital defects have been described in newborns whose mothers received fluconazole therapy for most or all of the first trimester.
The use of the drug in pregnant women is not advisable, with the exception of severe or life-threatening forms of fungal infections, if the expected benefit to the mother outweighs the possible risk to the fetus.
Women of childbearing age should use contraception.
Fluconazole is found in breast milk in the same concentration as in plasma, so its use during lactation is contraindicated.
Contraindications
Contraindications
• Simultaneous use of terfenadine (against the background of repeated doses of fluconazole at a dose of 400 mg/day or more) (see section “Interaction with other drugs”);
• Hypersensitivity to fluconazole and other components of the drug or azole compounds similar in structure;
• Children under 18 years of age;
• Lactation period (see section “Use during pregnancy and breastfeeding”);
• Concomitant use with drugs that increase the QT interval and are metabolized by the CYP3A4 isoenzyme, such as cisapride, astemizole, erythromycin, pimozide, quinidine and amiodarone (see section “Interaction with other drugs”);
• Galactose intolerance, lactase deficiency, glucose-galactose malabsorption.
With caution
• Impaired liver function indicators;
• Concomitant use of potentially hepatotoxic drugs;
• Alcoholism;
• Proarrhythmogenic conditions in patients with multiple risk factors (organic heart disease, electrolyte imbalance, concomitant use of drugs that cause arrhythmias);
• Renal dysfunction;
• The appearance of a rash during the use of fluconazole in patients with superficial fungal infection and invasive/systemic fungal infections;
• Simultaneous use of terfenadine and fluconazole at a dose of less than 400 mg/day.
Side Effects
Side Effects
The classification of adverse reactions by organs and systems is presented with an indication of the frequency of their occurrence: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (< 1/10000), including isolated reports, the frequency of which is unknown (the frequency cannot be estimated from the available data). The drug is usually very well tolerated. In clinical and post-marketing (*) studies of fluconazole, the following adverse reactions were noted:
Nervous system disorders: often – headache; uncommon – dizziness*, convulsions*, changes in taste*, paresthesia, insomnia, drowsiness; rarely – tremor.
Gastrointestinal disorders: often – abdominal pain, diarrhea, vomiting*, nausea; uncommon – flatulence, dyspepsia*, dry oral mucosa, constipation.
Disorders of the liver and biliary tract: often – increased serum activity of aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), alkaline phosphatase; uncommon – jaundice*, cholestasis, increased bilirubin concentration; rarely – hepatotoxicity, in some cases fatal, liver dysfunction*, hepatitis*, hepatocellular necrosis*, hepatocellular damage.
Skin disorders and
subcutaneous fat: often – rash; uncommon – skin itching, urticaria, increased sweating, drug rash; rarely – exfoliative skin lesions*, including Stevens-Johnson syndrome and toxic epidermal necrolysis, acute generalized exanthematous pustulosis, facial edema, alopecia*.
Blood and lymphatic system disorders*: rarely – leukopenia, including neutropenia and agranulocytosis, thrombocytopenia, anemia.
Immune system disorders*: rarely – anaphylaxis (including angioedema, facial edema).
Disorders of the cardiovascular system*: rarely – an increase in the duration of the QT interval on the ECG, ventricular tachycardia of the “pirouette” type (see section “Special Instructions”).
Metabolic disorders*: rarely – increased concentrations of cholesterol and triglycerides in the blood plasma, hypokalemia.
Musculoskeletal disorders: infrequently – myalgia.
Other: infrequently – weakness, asthenia, increased fatigue, fever, vertigo.
In some patients, especially those with serious illnesses such as HIV infection or cancer, changes in blood counts, renal and liver function have been observed during treatment with fluconazole and related drugs, but the clinical significance of these changes and their relationship to treatment has not been established.
If any of the above
in the instructions the side effects are aggravated, or any other are noted
side effects not listed in the instructions should be reported immediately
this doctor.
Interaction
Interaction
Single or multiple doses of fluconazole at a dose of 50 mg do not affect the metabolism of phenazone (Antipyrine) when taken once.
Concomitant use of fluconazole with the following drugs is contraindicated:
Cisapride. With the simultaneous use of fluconazole and cisapride, adverse reactions from the heart are possible, including ventricular tachysystolic arrhythmia of the “pirouette” type (torsade de pointes). The use of fluconazole at a dose of 200 mg once a day and cisapride at a dose of 20 mg 4 times a day leads to a marked increase in plasma concentrations of cisapride and an increase in the QT interval on the ECG.
The simultaneous use of cisapride and fluconazole is contraindicated.
Terfenadine. With the simultaneous use of azole antifungals and terfenadine, serious arrhythmias may occur as a result of an increase in the QT interval. When taking fluconazole at a dose of 200 mg/day, an increase in the QT interval has not been established.
However, the use of fluconazole in doses of 400 mg/day and above causes a significant increase in the concentration of terfenadine in the blood plasma. Concomitant use of fluconazole in doses of 400 mg/day or more with terfenadine is contraindicated (see section “Contraindications”). Treatment with fluconazole in doses less than 400 mg/day in combination with terfenadine should be carried out under close monitoring.
Astemizole. The simultaneous use of fluconazole with astemizole or other drugs whose metabolism is carried out by the cytochrome P450 system may be accompanied by an increase in serum concentrations of these drugs. Elevated concentrations of astemizole in blood plasma can lead to prolongation of the QT interval and, in some cases, to the development of ventricular tachysystolic arrhythmias of the torsade de pointes type. The simultaneous use of astemizole and fluconazole is contraindicated.
Pimozide. Although appropriate in vitro or in vivo studies have not been conducted, the simultaneous use of fluconazole and pimozide may lead to inhibition of the metabolism of pimozide.
In turn, an increase in plasma concentrations of pimozide can lead to a prolongation of the QT interval and, in some cases, the development of ventricular tachysystolic arrhythmia of the “pirouette” type (torsade de pointes). The simultaneous use of pimozide and fluconazole is contraindicated.
Quinidine. Although adequate in vitro or in vivo studies have not been conducted, concomitant use of fluconazole and quinidine may also lead to inhibition of quinidine metabolism. The use of quinidine is associated with prolongation of the QT interval and, in some cases, with the development of ventricular tachysystolic arrhythmias of the “torsade de pointes” type. The simultaneous use of quinidine and fluconazole is contraindicated.
Erythromycin. Concomitant use of fluconazole and erythromycin potentially leads to an increased risk of cardiotoxicity (QT prolongation, torsade de pointes) and, consequently, sudden cardiac death. The simultaneous use of fluconazole and erythromycin is contraindicated.
Amiodarone. Concomitant use of fluconazole and amiodarone may result in inhibition of amiodarone metabolism. Amiodarone use has been associated with QT prolongation. The simultaneous use of fluconazole and amiodarone is contraindicated (see section “Contraindications”).
Caution and possible dosage adjustments should be used when the following drugs are used concomitantly with fluconazole:
Drugs that affect fluconazole:
Hydrochlorothiazide. Simultaneous repeated use of fluconazole and hydrochlorothiazide can lead to an increase in the concentration of fluconazole in the blood plasma by 40%. An effect of this severity does not require a change in the fluconazole dosage regimen in patients receiving concomitant diuretics, but the doctor should take this into account.
Rifampicin. The combination with rifampicin leads to a decrease in AUC (area under the concentration-time curve) by 25% and a shortening of the plasma half-life of fluconazole by 20%. Therefore, in patients receiving concomitant rifampicin, it is necessary to consider the advisability of increasing the dose of fluconazole.
Drugs affected by fluconazole:
Fluconazole is a potent inhibitor of the cytochrome P 450 isoenzyme CYP2C9 and CYP2C19 and a moderate inhibitor of the CYP3A4 isoenzyme. In addition to the effects listed below, there is a risk of increased plasma concentrations of other drugs metabolized by the isoenzymes CYP2C9, CYP2C19 and CYP3A4 when taken simultaneously with fluconazole.
In this regard, caution should be exercised when using the following drugs simultaneously, and if such combinations are necessary, patients should be under close medical supervision. It should be taken into account that the inhibitory effect of fluconazole persists for 4-5 days after discontinuation of the drug due to the long half-life.
Alfentanil. There is a decrease in clearance and volume of distribution, and an increase in the half-life of alfentanil. This may be due to inhibition of the CYP3A4 isoenzyme by fluconazole. Alfentanil dosage adjustment may be required.
Amitriptyline, nortriptyline. Increase the effect. Concentrations of 5-nortriptyline and/or S-amitriptyline can be measured at the start of combination therapy with fluconazole and one week after the start of treatment. If necessary, the dose of amitriptyline/nortriptyline should be adjusted.
Amphotericin B. In studies in mice (including immunosuppressed mice), the following results were observed: a small additive antifungal effect in systemic infection with C. albicans, no interaction in intracranial infection with Cryptococcus neoformans, and antagonism in systemic infection with A. fumigatus. The clinical significance of these results is unclear.
Anticoagulants. When fluconazole, like other antifungal agents (azole derivatives), is used with warfarin, the prothrombin time increases (by an average of 12%).
Bleeding may develop (hematomas, bleeding from the nose and gastrointestinal tract, hematuria, melena). In patients receiving coumarin anticoagulants, prothrombin time must be constantly monitored during therapy and for 8 days after simultaneous use. The advisability of adjusting the warfarin dose should also be assessed.
Azithromycin. With simultaneous oral use of fluconazole in a single dose of 800 mg with azithromycin in a single dose of 1200 mg, no pronounced pharmacokinetic interaction has been established between both drugs.
Benzodiazepines (short-acting). After oral administration of midazolam, fluconazole significantly increases midazolam concentrations and psychomotor effects, and this effect is more pronounced after fluconazole is administered orally than when administered intravenously. If concomitant therapy with benzodiazepines is necessary, patients taking fluconazole should be monitored to assess the appropriateness of an appropriate reduction in the benzodiazepine dose.
When coadministered with a single dose of triazolam, fluconazole increases the AUC of triazolam by approximately 50%, Cmax by 25-50% and half-life by 25-50% due to inhibition of triazolam metabolism. Triazolam dose adjustment may be necessary.
Carbamazepine. Fluconazole inhibits the metabolism of carbamazepine and increases its serum concentration by 30%. The risk of carbamazepine toxicity must be taken into account. The need for carbamazepine dose adjustment based on concentration/effect should be assessed.
Nevirapine: Coadministration of fluconazole and nevirapine increases nevirapine exposure by approximately 100% compared with control data for nevirapine alone. Due to the risk of increased excretion of nevirapine during concomitant use of drugs, some precautions and careful monitoring of patients are necessary.
Calcium channel blockers. Some calcium channel antagonists (nifedipine, isradipine, amlodipine, verapamil and felodipine) are metabolized by the CYP3A4 isoenzyme. Fluconazole increases the systemic exposure of calcium channel antagonists. Monitoring for side effects is recommended.
Cyclosporine. It is recommended to monitor the concentration of cyclosporine in the blood in patients receiving fluconazole, since in patients with a kidney transplant, taking fluconazole at a dose of 200 mg/day leads to a slow increase in the concentration of cyclosporine in plasma. However, with repeated doses of fluconazole at a dose of 100 mg/day, no changes in cyclosporine concentrations were observed in bone marrow recipients.
Cyclophosphamide. With the simultaneous use of cyclophosphamide and fluconazole, an increase in serum concentrations of bilirubin and creatinine is observed. A combination of drugs is possible taking into account the risk of these disorders.
Fentanyl. There has been a report of one death possibly related to the concomitant use of fentanyl and fluconazole. The disturbances are believed to be related to fentanyl intoxication. Fluconazole has been shown to significantly prolong the clearance time of fentanyl. It should be borne in mind that an increase in the concentration of fentanyl can lead to depression of respiratory function.
Halofantrine. Fluconazole may increase plasma concentrations of halofantine due to inhibition of the CYP3A4 isoenzyme. When used simultaneously with fluconazole, as well as with other azole antifungal drugs, the development of ventricular tachysystolic arrhythmia of the “pirouette” type is possible, so their combined use is not recommended.
HMC-CoA reductase inhibitors. When fluconazole is used concomitantly with HMC-CoA reductase inhibitors metabolized by the CYP3A4 isoenzyme (such as atorvastatin and simvastatin) or the CYP2D6 isoenzyme (fluvastatin), the risk of developing myopathy and rhabdomyolysis increases. If simultaneous therapy with these drugs is necessary, patients should be monitored to identify symptoms of myopathy and rhabdomyolysis.
It is necessary to monitor the concentration of creatinine kinase. If there is a significant increase in creatinine kinase concentrations or if myopathy or rhabdomyolysis is diagnosed or suspected, therapy with HMC-CoA reductase inhibitors should be discontinued.
Losartan. Fluconazole inhibits the metabolism of losartan to its active metabolite (E-31 74), which is responsible for most of the effects associated with angiotensin-II receptor antagonism. Regular monitoring of blood pressure is necessary.
Methadone: Fluconazole may increase plasma concentrations of methadone. Methadone dosage adjustment may be necessary.
Nonsteroidal anti-inflammatory drugs (NSAIDs). Cmax and AUC of flurbiprofen increase by 23% and 81%, respectively. Same as Cmax
and AUC of the pharmacologically active isomer [S-(+)-ibuprofen] increased by 15% and 82%, respectively, when fluconazole was coadministered with racemic ibuprofen (400 mg).
With simultaneous use of fluconazole at a dose of 200 mg/day and celecoxib at a dose of 200 mg, the Cmax and AUC of celecoxib increased by 68% and 134%, respectively. In this combination, it is possible to reduce the dose of celecoxib by half.
Despite the lack of targeted studies, fluconazole may increase the systemic exposure of other NSAIDs metabolized by the CYP2C9 isoenzyme (naproxen, lornoxicam, meloxicam, diclofenac). NSAID dose adjustment may be necessary.
When NSAIDs and fluconazole are used concomitantly, patients should be closely monitored medically to identify and monitor NSAID-related adverse events and toxicities.
Oral contraceptives. With simultaneous use of a combined oral contraceptive with fluconazole at a dose of 50 mg, no significant effect on hormone levels has been established. With a daily dose of 200 mg fluconazole, the AUCs of ethinyl estradiol and levonorgestrel increased by 40% and 24%, respectively. When fluconazole 300 mg was administered once weekly, the AUCs of ethinyl estradiol and norethindrone increased by 24% and 13%, respectively. Thus, repeated use of fluconazole at the indicated doses is unlikely to affect the effectiveness of the combined oral contraceptive.
Phenytoin. Concomitant use of fluconazole and phenytoin may lead to an increase in plasma phenytoin concentrations to a clinically significant extent. Therefore, if it is necessary to use these drugs together, it is necessary to monitor phenytoin concentrations with dose adjustment in order to maintain drug levels within the therapeutic interval.
Prednisone. There is a report of the development of acute adrenal insufficiency in a patient after liver transplantation when fluconazole was discontinued after a three-month course of therapy. Presumably, discontinuation of fluconazole therapy caused an increase in the activity of the CYP3A4 isoenzyme, which led to increased metabolism of prednisone.
Patients receiving combination therapy with prednisone and fluconazole should be under close medical supervision when discontinuing fluconazole to assess the condition of the adrenal cortex.
Rifabutin. The simultaneous use of fluconazole and rifabutin can lead to an increase in serum concentrations of the latter by up to 80%. Cases of uveitis have been described with the simultaneous use of fluconazole and rifabutin. Patients receiving rifabutin and fluconazole simultaneously should be carefully monitored.
Saquinavir. AUC increases by approximately 50%, Cmax by 55%. The clearance of saquinavir is reduced by approximately 50% due to inhibition of the hepatic metabolism of CYP3A4 and P-glycoprotein. Dose adjustment of saquinavir may be necessary.
Sirolimus. An increase in the concentration of sirolimus in the blood plasma is presumably due to inhibition of the metabolism of sirolimus through inhibition of the CYP3A4 isoenzyme and P-glycoprotein. This combination can be used with appropriate dose adjustment of sirolimus depending on the effect/concentration.
Sulfonylurea drugs. Fluconazole increases the plasma half-life of oral hypoglycemic drugs – sulfonylurea derivatives (chlorpropamide, glibenclamide, glipizide, tolbutamide). The combined use of fluconazole and oral hypoglycemic agents is allowed, but the doctor must keep in mind the possibility of developing hypoglycemia. Regular monitoring of blood glucose and, if necessary, dose adjustment of sulfonylurea drugs are necessary.
Tacrolimus. The use of fluconazole and tacrolimus (orally) leads to an increase in serum concentrations of the latter by 5 times due to inhibition of the metabolism of tacrolimus occurring in the intestine through the CYP3A4 isoenzyme. No significant changes in the pharmacokinetics of the drugs were observed when tacrolimus was administered intravenously. Cases of nephrotoxicity have been described. Patients receiving oral tacrolimus and fluconazole concomitantly should be monitored closely. The dose of tacrolimus should be adjusted depending on the degree of increase in its concentration in the blood.
Theophylline. When used simultaneously with fluconazole at a dose of 200 mg for 14 days, the average rate of plasma clearance of theophylline is reduced by 18%. When prescribing fluconazole to patients taking high doses of theophylline or to patients at increased risk of developing theophylline toxicity, monitor for symptoms of theophylline overdose and, if necessary, adjust therapy accordingly.
Vinca alkaloid. Although targeted studies are lacking, it is suspected that fluconazole may increase plasma concentrations of vinca alkaloids (eg, vincristine and vinblastine) and thus lead to neurotoxicity, which may be due to inhibition of CYP3A4.
Vitamin A. There is a report of one case of the development of adverse reactions from the central nervous system (CNS) in the form of pseudotumor cerebri with the simultaneous use of all-trans retinoic acid and fluconazole, which disappeared after discontinuation of fluconazole. The use of this combination is possible, but one should remember the possibility of adverse reactions from the central nervous system.
Zidovudine. In patients receiving a combination of fluconazole and zidovudine, there is an increase in the Cmax and AUC of zidovudine by 84% and 74%, respectively, which is caused by a decrease in the metabolism of the latter to its main metabolite. Before and after therapy with fluconazole at a dose of 200 mg/day for 15 days in patients with AIDS and ARC (AIDS-related complex), a significant increase in the AUC of zidovudine (20%) was found. Patients receiving this combination should be monitored for side effects of zidovudine.
Voriconazole (inhibitor of the isoenzyme CYP2C9, CYP2C19 and CYP3A4). Concomitant use of voriconazole (400 mg twice daily on day 1, then 200 mg twice daily for 2.5 days) and fluconazole (400 mg on day 1, then 200 mg daily for 4 days) increased voriconazole concentrations and AUC by 57% and 79%, respectively.
It has been shown that this effect persists when the dose and/or frequency of administration of any of the drugs is reduced. Concomitant use of voriconazole and fluconazole is not recommended.
Tofacitinib. The exposure of tofacitinib is increased when it is co-administered with drugs that are both moderate inhibitors of the CYP3A4 isoenzyme and strong inhibitors of the CYP2C19 isoenzyme (for example, fluconazole). A dose adjustment of tofacitinib may be necessary.
Ivacaftor. When coadministered with ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) stimulator, there was a 3-fold increase in ivacaftor exposure and a 1.9-fold increase in hydroxymethyl-ivacaftor (M1) exposure. For patients taking CYP3A inhibitors concomitantly, such as fluconazole and erythromycin, a dose reduction of ivacaftor to 150 mg once daily is recommended.
Studies of the interaction of oral forms of fluconazole when taken simultaneously with food, cimetidine, antacids, as well as after total body irradiation in preparation for bone marrow transplantation showed that these factors do not have a clinically significant effect on the absorption of fluconazole.
The listed interactions were established with repeated use of fluconazole; There are no known drug interactions resulting from a single dose of fluconazole.
Doctors should
take into account that interactions with other drugs are not specifically
has been studied, but it is possible.
Overdose
Overdose
Symptoms: hallucinations, paranoid behavior.
Treatment: symptomatic, gastric lavage, forced diuresis.
Hemodialysis within 3 hours reduces plasma concentrations by approximately 50%.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | capsules |
| Brand | Pharmstandard-Leksredstva |
Other forms…
Related products
Buy Flucostat, 150 mg capsules 2 pcs with delivery to USA, UK, Europe and over 120 other countries.