No products in the cart.
Description
Progressive hormone-dependent prostate cancer.
Indications
Indications
Progressive hormone-dependent prostate cancer.
Special instructions
Special instructions
The use of degarelix has not been studied in patients with a history of a QTc interval greater than 450 ms, in patients with risk factors for the development of torsades de pointes (TdP), or in combination with drugs that can prolong the QT interval. If it is necessary to use degarelix in such cases, the expected benefits and risks of therapy should be carefully assessed.
The therapeutic efficacy of degarelix should be assessed by clinical parameters and serum PSA levels. Testosterone suppression occurs immediately after the initial dose, with plasma testosterone levels falling to medical castration testosterone levels (<0.5 ng/ml) in 96% of patients after three days, and in 100% of patients after a month. Long-term, up to 1 year, maintenance therapy provides testosterone suppression (<0.5 ng/ml) in 97% of patients. If the therapeutic effect is not clearly expressed in the patient, you should make sure that the level of testosterone in the blood serum remains sufficiently reduced. Degarelix does not cause fluctuations in testosterone levels, so there is no need to use antiandrogen drugs at the beginning of treatment.
There is no need to change the dose for elderly patients or for patients with mild or moderate hepatic or renal impairment. Degarelix should be used with caution in patients with severe hepatic or renal impairment, because it has not been studied in this category of patients.
Changes in bone density with degarelix have not been studied, but decreased bone density has been observed in patients who have had orchiectomy or taken GnRH agonists. Therefore, due to testosterone suppression caused by degarelix, a decrease in bone density is possible.
The effect of degarelix on insulin and blood glucose levels has not been studied. However, patients who have undergone orchiectomy or are taking GnRH agonists have a decrease in insulin sensitivity to glucose and possibly the development or exacerbation of diabetes mellitus. Therefore, regular monitoring of blood glucose levels is recommended during treatment with degarelix.
Use in pediatrics
There are no valid indications for the use of degarelix in children or adolescents.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
When using degarelix, fatigue and dizziness are possible, which may affect the patient’s ability to drive vehicles and other potentially hazardous activities.
Active ingredient
Active ingredient
Degarelix
Composition
Composition
1 bottle contains:
Active ingredients:
degarelix 120 mg.
The bottle contains 120 mg of lyophilisate.
There are 2 bottles in a cardboard package.
Contraindications
Contraindications
Hypersensitivity to degarelix.
Side Effects
Side Effects
From the nervous system: often (≥ 1%, < 10%) - insomnia, dizziness, headaches; rarely (≥ 0.01%, < 0.1%) - decreased libido, depression, irritability, ringing in the ears.
From the cardiovascular system: very often (≥ 10%) – hot flashes; rarely (≥ 0.01%, < 0.1%) - 1st degree AV block, increased QT interval, increased blood pressure, vasovagal syncope, varicose veins.
From the digestive system: very often (≥ 10%) – nausea; often (≥ 1%, < 10%) - diarrhea, vomiting, flatulence, dry mouth, constipation, increased activity of liver transaminases; rarely (≥ 0.01%, < 0.1%) - loss of appetite.
From the respiratory system: rarely (≥ 0.01%, < 0.1%) - cough.
From the hematopoietic system: rarely (≥ 0.01%, < 0.1%) - anemia.
Dermatological reactions: often (≥ 1%, < 10%) - increased sweating (including night sweats); rarely (≥ 0.01%, < 0.1%) - urticaria, skin hyperpigmentation, hair loss, softening of nails, rash, erythema.
From the musculoskeletal system: rarely (≥ 0.01%, < 0.1%) - muscle pain, muscle weakness.
From the reproductive system: rarely (≥ 0.01%, < 0.1%) - erectile dysfunction, testicular atrophy, gynecomastia, lower abdominal pain, prostate tenderness, retrograde ejaculation, testicular tenderness.
From the urinary system: rarely (≥ 0.01%, < 0.1%) - frequent urination, urgent urge to urinate, renal failure.
Metabolism: 6% – severe hypokalemia (≥ 5.8 mmol/l); 2% – decrease in creatinine level (≥177 µmol/l); 15% – increase in blood urea nitrogen (≥10.7 mmol/l).
Other: very often (≥ 10%) – irritation at the injection site; often (≥ 1%, < 10%) - chills, fever, weakness, fatigue, colds; rarely (≥ 0.01%, < 0.1%) - hypersensitivity reactions, swelling at the injection site.
Interaction
Interaction
Since QT interval prolongation is possible with the use of degarelix, the need for concomitant use of degarelix and drugs that cause QT prolongation or ventricular tachycardia (e.g., quinidine, disopyramide), antipsychotics, antiarrhythmic drugs (e.g., amiodarone, sotalol, dofetilide, ibutilide), as well as methadone, cisapride and moxifloxacin should be assessed.
A clinically significant pharmacokinetic interaction between degarelix and agents that affect the CYP450 enzyme system is unlikely.
Overdose
Overdose
Symptoms: There are no data on symptoms of acute degarelix overdose.
Treatment: in case of overdose, the patient should be monitored and, if necessary, supportive therapy should be used.
Storage conditions
Storage conditions
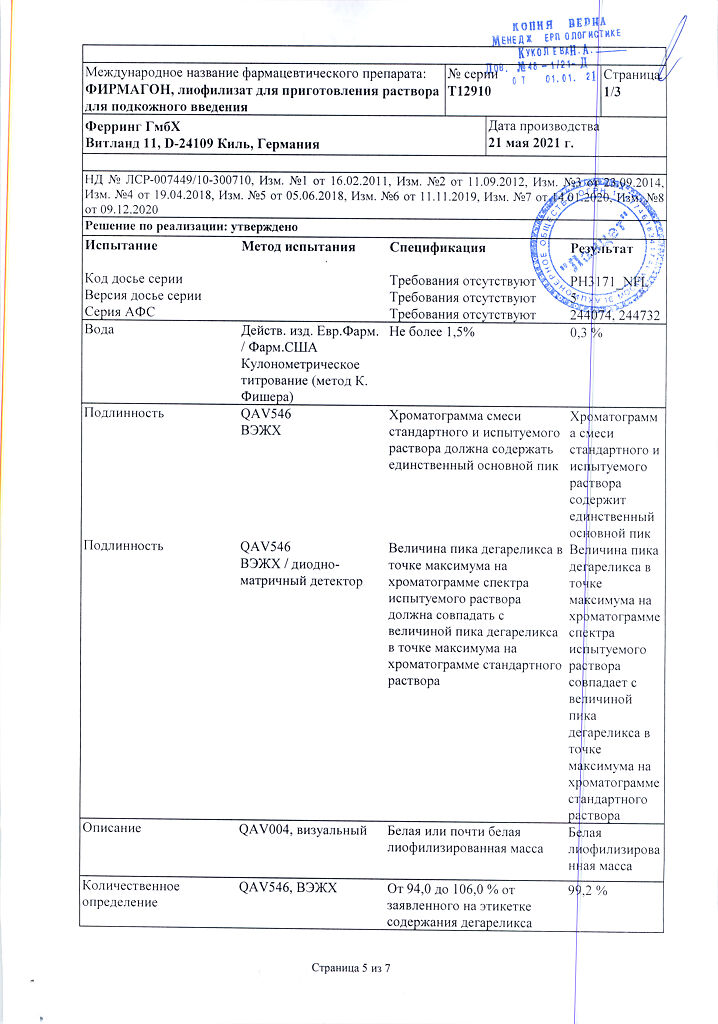
Store at a temperature not exceeding 25 °C. Store the prepared solution at a temperature not exceeding 25 °C.
Shelf life
Shelf life
2 years. Store the prepared solution for no more than 2 hours.
Manufacturer
Manufacturer
Ferring GmbH, Germany
Additional information
| Shelf life | 2 years. Ready solution should be stored for no more than 2 hours. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 °C. Store the prepared solution at a temperature not exceeding 25 °C. |
| Manufacturer | Ferring GmbH, Germany |
| Medication form | lyophilizate |
| Brand | Ferring GmbH |
Related products
Buy Firmagon, lyophilizate 120 mg 2 pcs with delivery to USA, UK, Europe and over 120 other countries.