No products in the cart.
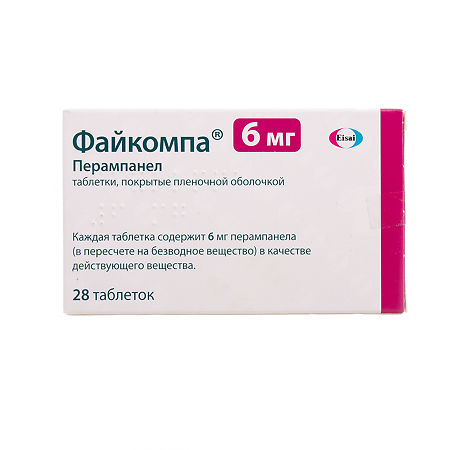
Ficompa, 6 mg 28 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Faicompa has an antiepileptic effect.
Pharmacodynamics
The mechanism of action. Perampanel is a first-in-class selective noncompetitive ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) glutamate receptor antagonist on postsynaptic neurons.
Glutamate is the main excitatory neurotransmitter in the CNS and plays an important role in the pathogenesis of several neurological diseases caused by neuronal overexcitation.
The activation of AMPA receptors by glutamate is thought to be responsible for the most rapid excitatory synaptic transmission in the brain.
In in vitro studies, perampanel did not compete with AMPA for binding to the AMPA receptor, but it was displaced from binding by noncompetitive AMPA receptor antagonists. This indicates that perampanel is a noncompetitive AMPA receptor antagonist.
In in vitro studies, perampanel inhibited AMPA-induced (but not N-methyl-D-aspartate (NMDA)-induced) increases in intracellular calcium concentration. Perampanel significantly increased the latency period in an AMPA-induced model of epileptic seizure in vivo.
The exact mechanism of the anticonvulsant effect of perampanel in humans is subject to further study.
Pharmacodynamic effects.
The pharmacokinetics and pharmacodynamics of perampanel were analyzed based on pooled data from three efficacy studies conducted in partial epileptic seizures. The magnitude of exposure to perampanel correlated with the severity of seizure frequency reduction.
The effect on psychomotor functions. At doses of 8 and 12 mg, perampanel worsened psychomotor functions in healthy volunteers in a dose-dependent manner when administered once and repeatedly. The effect of perampanel on complex psychomotor functions, such as driving, was enhanced by alcohol intake. Characteristics of psychomotor functions returned to baseline values within 2 weeks after discontinuation of perampanel.
The effect on cognitive function. When assessed in a series of standard tests of the effects of perampanel on the speed of response to external stimuli and memory in healthy volunteers, no effect was noted with both single and multiple doses of the drug in doses up to 12 mg/day.
Impact on mood and response speed to external stimuli. The speed of reaction to external stimuli (arousal) in healthy volunteers receiving perampanel in doses from 4 to 12 mg/day decreased in a dose-dependent manner. Mood deterioration in the volunteers was observed only on 12 mg/day, the mood changes were insignificant and reflected an overall decrease in the rate of response to external stimuli.
Multiple administration of perampanel at a daily dose of 12 mg increased the worsening effect of alcohol on attention and responsiveness, and increased the severity of irritability, confusion, and depression on a 5-point Mood Profile scale.
The effect on electrophysiological parameters of the heart. Perampanel at daily doses of up to 12 mg did not prolong the QTc interval and had no dose-dependent or clinically significant effect on QRS complex duration.
Clinical efficacy and safety. The efficacy of Ficompa® for partial seizures was established in three 19-week randomized, double-blind, placebo-controlled, multicenter studies in adults and adolescents with partial seizures with or without secondary generalization not adequately controlled by other (one to a combination of three) antiepileptic drugs (PEDs). Patients had to have more than 5 seizures during the 6-week baseline period; the period without seizures was not to exceed 25 days. In all three studies, patients had a mean duration of illness of 21.06 years. Between 85.3 and 89.1% of patients were taking 2 or 3 PEPs with or without concomitant vagus nerve stimulation.
The first two studies compared Ficompa® at daily doses of 8 and 12 mg and the third study at daily doses of 2, 4, and 8 mg with placebo.
In all three studies, after a 6-week baseline period conducted before randomization and necessary to establish baseline epileptic seizure rates, patients were randomized and received doses matched to randomized values. During the dose-fitting period, treatment in all three trials began at a dose of 2 mg/day, which was increased weekly by 2 mg/day until the target dose was reached. Patients with intolerable side effects could remain on the same dose or their dose was reduced to the previously well tolerated dose. In all three studies, the dose adjustment period was followed by a maintenance period of 13 weeks, during which patients had to receive a steady dose of Ficomp.
According to the combined results of the three studies, the 50% response rate was 19% for the placebo group, 29% for the 4 mg dose, 35% for the 8 mg dose, and 35% for the 12 mg dose, respectively. A statistically significant reduction in seizure frequency over 28 days compared to placebo was shown for doses of 4 to 12 mg/day when taken once.
These results indicate that perampanel doses of 4 to 12 mg once daily as adjunctive therapy are significantly more effective in this patient group than placebo.
A clinically significant improvement in seizure control was observed with a single dose of 4 mg/day of Feicomp, and increased when the daily dose was increased to 8 mg. When the daily dose was increased to 12 mg, no additional improvement in efficacy was observed compared with the 8 mg dose in the entire patient population. Increased efficacy of Ficompa at the 12 mg dose was observed only in patients who were resistant to the 8 mg dose.
A clinically significant reduction in seizure frequency relative to placebo was achieved as early as week 2 after reaching the daily dose of 4 mg.
The open extended study
97% (1,186) of patients who completed randomized trials were recruited to participate in an open extended study in which they took perampanel for at least 1 year at a mean daily dose of 10.05 mg.
These three baseline double-blind, placebo-controlled phase III studies enrolled 143 adolescents aged 12 to 18 years. The results obtained in adolescents were similar to those of adult patients.
Pharmacokinetics
The pharmacokinetics of perampanel were studied in healthy volunteers aged 18 to 79 years, in adults and adolescents with partial epileptic seizures, in adults with Parkinson’s disease, diabetic nephropathy and multiple sclerosis, and in patients with hepatic impairment.
Intake. When administered orally, perampanel is quickly and completely absorbed, the effect of first passage through the liver is negligible. Food intake has no effect on the degree of absorption, but slows down its rate. Compared with fasting when concomitant intake of the drug with food, the Cmax of perampanel in plasma is reduced and its time of attainment is increased by 2 hours.
Distribution. Data from in vitro studies indicate that perampanel is approximately 95% bound to plasma proteins. In vitro, perampanel has been shown to be neither a substrate nor a significant inhibitor of organic anion transport peptides (OATP) 1B1 and 1B3, organic anion transporters (OAT) 1, 2, 3 and 4, organic cation transporters (OCT) 1, 2 and 3, and P-glycoprotein and breast cancer resistance protein (BCRP).
Metabolism. Perampanel is largely metabolized by primary oxidation and subsequent glucuronidation.
According to in vitro studies with recombinant cytochrome P450 in human liver microsomes, primary oxidative metabolism is mediated by CYP3A4 isoenzymes. However, the metabolism of perampanel is not fully understood, its other pathways are not excluded.
After the use of radioactively labeled perampanel, only trace amounts of its metabolites are determined in plasma.
Evacuation. After administration of radioactively labeled perampanel in healthy elderly volunteers, 30% of the radioactive label was detected in the urine and 70% in the feces. The radioactive label excreted was mainly a mixture of oxidized and conjugated metabolites. In a population pharmacokinetic analysis of pooled data from 19 Phase I clinical trials, the mean T1/2 of perampanel was 105 h. When concomitantly used with carbamazepine, a potent inducer of the CYP3A4 isoenzyme, the T1/2 of perampanel was 25 h.
Linearity/Nonlinearity. In healthy volunteers, the plasma concentration of perampanel increases in direct proportion to the dose between 2 and 12 mg. In a population pharmacokinetic analysis in patients with partial seizures receiving perampanel at doses up to 12 mg/day in placebo-controlled clinical trials, a linear relationship was established between the dose amount and the plasma concentration of perampanel.
Perampanel use in special patient groups
Patients with hepatic impairment. The pharmacokinetics of perampanel after a single dose of 1 mg were evaluated in 12 patients with mild to moderate hepatic impairment (Child-Pugh grades A and B) and demographically matched 12 healthy volunteers. The mean apparent clearance of unbound perampanel in mild hepatic insufficiency was 188 mL/min versus 338 mL/min in healthy volunteers and 120 mL/min (versus 392 mL/min) in moderate. The T1/2 in patients with hepatic insufficiency was prolonged: up to 306 hours in mild versus 125 hours in healthy volunteers, and up to 295 hours in moderate versus 139 hours .
Patients with renal insufficiency. The pharmacokinetics of perampanel in patients with renal impairment have not been studied separately. Elimination of perampanel occurs almost exclusively by formation of metabolites followed by their rapid excretion. Only trace amounts of perampanel metabolites are detected in plasma. In a population pharmacokinetic analysis in patients with partial seizures and creatinine Cl 39-160 mL/min receiving perampanel at doses up to 12 mg/day in placebo-controlled studies, no relationship between perampanel clearance and creatinine clearance was observed.
The effect of sex. In a population pharmacokinetic analysis of patients with partial seizures who received perampanel at doses up to 12 mg/d in placebo-controlled trials, perampanel clearance in women (0.605 l/h) was 17% lower than in men (0.73 l/h).
Patients of advanced age (≥65 years). In a population pharmacokinetic analysis in patients aged 12 to 74 years with partial seizures who received perampanel in placebo-controlled studies at doses up to 12 mg/day, there was no significant effect of age on perampanel clearance.
Patients in pediatric patients. In a population pharmacokinetic analysis, there were no significant differences from the general population in pediatric patients enrolled in phase III clinical trials.
Drug interaction studies
In vitro drug interaction assessment
Inhibition of enzymes involved in drug metabolism. Among the major cytochromes and UDF-glucuronyltransferases of the liver, perampanel (30 μmol/L) weakly inhibited the CYP2C8 and UGT1A9 isoenzymes in vitro in experiments on human liver microsomes.
Induction of enzymes involved in drug metabolism. Compared with positive controls (including phenobarbital and rifampicin), perampanel weakly induced the CYP2B6 (30 μmol/L) and CYP3A4/5 (≥3 μmol/L) isoenzymes among major cytochrome and UDF-glucuronyltransferases in human hepatocyte culture.
Indications
Indications
Fycompa is indicated as an adjuvant for the treatment of partial seizures in patients with epilepsy aged 12 years and older with or without secondary generalized seizures.
Pharmacological effect
Pharmacological effect
Faycompa has an antiepileptic effect.
Pharmacodynamics
Mechanism of action. Perampanel is a first-in-class selective, noncompetitive antagonist of ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) glutamate receptors on postsynaptic neurons.
Glutamate is the main excitatory neurotransmitter in the central nervous system, playing an important role in the pathogenesis of a number of neurological diseases caused by overexcitation of neurons.
It is assumed that activation of AMPA receptors by glutamate is responsible for the most rapid excitatory synaptic transmission in the brain.
In in vitro studies, perampanel did not compete with AMPA for binding to the AMPA receptor, but was displaced from binding by noncompetitive AMPA receptor antagonists. This indicates that perampanel is a non-competitive AMPA receptor antagonist.
In in vitro studies, perampanel inhibited AMPA-induced (but not N-methyl-D-aspartate (NMDA)-induced) increases in intracellular calcium concentrations. Perampanel significantly increased latency in an AMPA-induced seizure model in vivo.
The exact mechanism of development of the anticonvulsant effect of perampanel in humans is subject to further study.
Pharmacodynamic effects.
Based on the summary data of three studies conducted on the effectiveness of partial epileptic seizures, an analysis of the pharmacokinetics and pharmacodynamics of perampanel was carried out. The magnitude of the effect of perampanel correlated with the severity of the reduction in seizure frequency.
Effect on psychomotor functions. At doses of 8 and 12 mg, perampanel, with single and multiple doses, dose-dependently worsened psychomotor functions in healthy volunteers. The effect of perampanel on complex psychomotor functions, such as driving, was enhanced by alcohol intake. Characteristics of psychomotor functions returned to baseline values within 2 weeks after discontinuation of perampanel.
Effect on cognitive function. When assessing in a series of standard tests the effect of perampanel on the speed of reaction to external influences and memory in healthy volunteers, no effect was noted, either with a single or repeated dose of the drug in doses of up to 12 mg/day.
Effect on mood and speed of reaction to external influences. The rate of reaction to external influence (excitation) in healthy volunteers receiving perampanel in doses from 4 to 12 mg/day decreased in a dose-dependent manner. A deterioration in the mood of the volunteers was observed only when taking 12 mg/day; the changes in mood were insignificant and reflected a general decrease in the speed of reaction to external influences.
Repeated administration of perampanel at a daily dose of 12 mg increased the worsening effect of alcohol on alertness and reaction time to external influences and increased the severity of irritability, confusion and depression according to the 5-point Mood Profile rating scale.
Effect on electrophysiological parameters of the heart. Perampanel in daily doses of up to 12 mg did not prolong the QTc interval and did not have any dose-dependent or clinically significant effect on the duration of QRS complexes.
Clinical efficacy and safety. The effectiveness of Fycompa® for partial seizures was established in three 19-week randomized, double-blind, placebo-controlled, multicenter studies in adults and adolescents with partial seizures with or without secondary generalization not adequately controlled by other (one to a combination of three) antiepileptic drugs (AEDs). During the 6-week baseline period, patients must have had more than 5 seizures, and the seizure-free period must not have exceeded 25 days. In all three studies, patients had a mean disease duration of 21.06 years. Between 85.3 and 89.1% of patients took 2 or 3 AEDs with or without concomitant vagus nerve stimulation.
The first two studies compared Fycompa® at daily doses of 8 and 12 mg, and the third at daily doses of 2, 4 and 8 mg to placebo.
In all three studies, after a 6-week pre-randomization baseline period to establish the baseline seizure frequency, patients were randomized to receive doses adjusted to the randomized values. During the dose adjustment period in all three studies, treatment began at a dose of 2 mg/day, which was increased weekly by 2 mg/day until the target dose was achieved. Patients with intolerable side effects could remain on the same dose or have their dose reduced to a previously well-tolerated dose. In all three studies, the dose titration period was followed by a maintenance period of 13 weeks during which patients were required to receive a constant dose of Fycompa.
According to the pooled results of the three studies, the 50% response rate was 19% for the placebo group, 29% for the 4 mg dose, 35% for the 8 mg dose and 35% for the 12 mg dose. A statistically significant reduction in seizure frequency at 28 days compared to placebo was shown for doses ranging from 4 to 12 mg/day as a single dose.
These results indicate that perampanel, administered in doses of 4 to 12 mg once daily as adjunctive therapy in this group of patients, is significantly more effective than placebo.
Clinically significant improvement in seizure control was observed with a single dose of 4 mg/day of Fycompa, and increased when the daily dose was increased to 8 mg. When the daily dose was increased to 12 mg, no additional increase in the effectiveness of the drug compared to the 8 mg dose was observed in the entire patient population. An increase in the effectiveness of Fycompa at a dose of 12 mg was observed only in patients resistant to a dose of 8 mg.
A clinically significant reduction in the frequency of attacks relative to placebo was achieved already in the 2nd week after reaching a daily dose of 4 mg.
Open-label extension study
97% (1186) of patients completing randomized studies were recruited to participate in an open-label extension study in which they received perampanel for at least 1 year at a mean daily dose of 10.05 mg.
These three pivotal, double-blind, placebo-controlled, phase III studies included 143 adolescents aged 12 to 18 years. The results obtained in adolescents were similar to those in adult patients.
Pharmacokinetics
The pharmacokinetics of perampanel were studied in healthy volunteers aged 18 to 79 years, in adults and adolescents with partial epileptic seizures, in adults with Parkinson’s disease, diabetic nephropathy and multiple sclerosis, as well as in patients with liver failure.
Suction. When taken orally, perampanel is quickly and completely absorbed; the first-pass effect through the liver is negligible. Eating does not affect the degree of absorption, but slows down its speed. Compared with administration on an empty stomach, when the drug is taken simultaneously with food, the Cmax of perampanel in plasma is reduced, and the time to reach it increases by 2 hours.
Distribution. Data from in vitro studies indicate that perampanel is approximately 95% bound to plasma proteins. In vitro, perampanel has been shown to be neither a substrate nor a significant inhibitor of organic anion transport peptides (OATP) 1B1 and 1B3, organic anion transporter (OAT) 1, 2, 3 and 4, organic cation transporter (OCT) 1, 2 and 3, and P-glycoprotein and breast cancer resistance protein (BCRP).
Metabolism. Perampanel is largely metabolized by primary oxidation and subsequent glucuronidation.
According to the results of in vitro studies with recombinant cytochrome P450 in human liver microsomes, primary oxidative metabolism is mediated by CYP3A4 isoenzymes. However, the metabolism of perampanel has not been fully studied; other pathways are not excluded.
After the use of radioactively labeled perampanel, only trace amounts of its metabolites are determined in the plasma.
Excretion. After administration of radiolabeled perampanel to healthy elderly volunteers, 30% of the radiolabel was detected in urine and 70% in feces. The isolated radiolabel was mainly a mixture of oxidized and conjugated metabolites. In a population pharmacokinetic analysis of summary data from 19 phase I clinical studies, the average T1/2 of perampanel was 105 hours. When used simultaneously with carbamazepine, which is a powerful inducer of the CYP3A4 isoenzyme, T1/2 of perampanel was 25 hours.
Linearity/Nonlinearity. In healthy volunteers, the plasma concentration of perampanel increases in direct proportion to the dose in the range from 2 to 12 mg. In a population pharmacokinetic analysis of patients with partial seizures receiving perampanel in doses up to 12 mg/day in placebo-controlled clinical trials, a linear relationship was established between the dose size and the plasma concentration of perampanel.
Use in special patient groups
Patients with liver failure. The pharmacokinetics of perampanel following a single 1 mg dose were assessed in 12 patients with mild to moderate hepatic impairment (Child-Pugh classes A and B) and demographically matched 12 healthy volunteers. The average apparent clearance of unbound perampanel in mild liver failure was 188 ml/min versus 338 ml/min in healthy volunteers and 120 ml/min (versus 392 ml/min) in moderate liver failure. T1/2 in patients with liver failure was prolonged: with mild degrees – up to 306 hours versus 125 hours in healthy volunteers, with moderate degrees – up to 295 hours versus 139 hours.
Patients with renal failure. The pharmacokinetics of perampanel in patients with renal failure have not been studied separately. Elimination of perampanel is carried out almost exclusively by the formation of metabolites followed by their rapid excretion. Only trace amounts of perampanel metabolites are found in plasma. In a population pharmacokinetic analysis in patients with partial seizures and creatinine Cl 39–160 ml/min who received perampanel in doses up to 12 mg/day during placebo-controlled studies, no relationship was noted between perampanel clearance and creatinine clearance.
Influence of gender. In a population pharmacokinetic analysis of patients with partial seizures receiving perampanel in doses up to 12 mg/day in placebo-controlled studies, the clearance of perampanel in women (0.605 L/h) was 17% lower than in men (0.73 L/h).
Elderly patients (≥65 years). In a population pharmacokinetic analysis of patients aged 12 to 74 years with partial-onset seizures receiving perampanel at doses up to 12 mg/day in placebo-controlled studies, no significant effect of age on the clearance of perampanel was found.
Pediatric patients. In a population pharmacokinetic analysis, no significant differences from the general population were found in adolescent patients participating in phase III clinical trials.
Drug interaction studies
In vitro drug interaction assessment
Inhibition of enzymes involved in drug metabolism. Among the main cytochromes and UDP-glucuronyltransferases of the liver, perampanel (30 µmol/l) weakly inhibited the CYP2C8 and UGT1A9 isoenzymes in vitro in experiments on human liver microsomes.
Induction of enzymes involved in drug metabolism. Compared with the positive control (including phenobarbital and rifampicin) among the main cytochromes and UDP-glucuronyltransferases in cultured human hepatocytes, perampanel weakly induced the isoenzymes CYP2B6 (30 μmol/L) and CYP3A4/5 (≥3 μmol/L).
Special instructions
Special instructions
Suicidal alertness
Active ingredient
Active ingredient
Perampanel
Composition
Composition
Active ingredient:
Pregnancy
Pregnancy
For women with preserved childbearing potential who do not use contraceptive methods, taking Fycompa is recommended only when absolutely necessary.
Data on the use of perampanel in pregnant women are significantly limited (<300 cases). No direct or indirect toxic effects were observed in animal reproductive toxicity studies. As a precaution, it is recommended to avoid the use of Fycompa during pregnancy.
Animal studies have shown that perampanel and/or its metabolites are excreted in breast milk. It is unknown whether perampanel is excreted into breast milk in humans, so a risk to the baby cannot be excluded.
Given the benefits of both breastfeeding for the child and therapy for the woman, it is necessary to either stop breastfeeding or refrain from taking/discontinue taking Fycompa during breastfeeding.
Effect on fertility
In animal studies, it was shown that in high doses (30 mg/kg), perampanel prolongs and disrupts the regularity of the estrous cycle, but these changes did not affect fertility and early fetal development. No effect on male fertility was found. The effect of perampanel on human fertility has not been studied.
Contraindications
Contraindications
Hypersensitivity to perampanel or any of the excipients of the drug.
Side Effects
Side Effects
Among patients with partial seizures who received perampanel in all clinical studies conducted, 72% took the drug for 6 months and 43% for more than 12 months.
Interaction
Interaction
Oral contraceptives
Overdose
Overdose
Clinical experience with perampanel overdose in humans is limited. In a report of an intentional overdose that could have resulted in doses up to 264 mg, the patient experienced altered consciousness, agitation, and aggressive behavior; recovery was without consequences.
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Eisai, Japan
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Eisai, Japan |
| Medication form | pills |
| Brand | Eisai |
Other forms…
Related products
Buy Ficompa, 6 mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.