No products in the cart.
Fervex for children, 8 pcs.
€11.97 €9.97
Description
Remedy for acute respiratory infections and “colds” (analgesic non-narcotic + vitamin + H1-histamine receptor blocker).
ATC code: N02VE51.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
Fervex® for children is a combined drug that contains paracetamol, pheniramine maleate and ascorbic acid.
Paracetamol is a non-steroidal anti-inflammatory drug, blocks cyclooxygenase, mainly in the central nervous system, affecting the centers of pain and thermoregulation; it has analgesic and antipyretic effects.
Paracetamol has analgesic and antipyretic effect.
Pheniramine maleate is a blocker of H1-histamine receptors. Narrowes nasal vessels, eliminates nasal mucous membrane edema and hyperemia of the nasal cavity, nasopharynx and sinuses.
Ascorbic acid is involved in the regulation of redox processes, reduces vascular permeability and increases the body’s resistance.
PHARMACOKINETICS:
Absorption of paracetamol is complete and rapid. Peak plasma concentrations are reached 30-60 minutes after ingestion. Distribution of paracetamol in tissues is rapid. Comparable concentrations of the drug in blood, saliva and plasma are achieved. The binding to plasma proteins is low, 10-25%. It penetrates through the blood-brain barrier.
Metabolism occurs in the liver, 80% reacts with glucuronic acid and sulfates to form inactive metabolites; 17% undergoes hydroxylation to form 8 active metabolites, which conjugate with glutathione to form inactive metabolites. In case of glutathione deficiency these metabolites can block enzyme systems of hepatocytes and cause their necrosis. CYP2E1 isoenzyme is also involved in metabolism of the drug. T 1/2 – 1-4 hours. It is excreted by the kidneys as metabolites, mainly conjugates. Less than 5% is excreted unchanged. The elimination half-life is about 2 hours.
Pheniramine maleate:
It is well absorbed in the digestive tract. Period of elimination from blood plasma is one to one and a half hours. It is eliminated from the body mainly through the kidneys.
Ascorbic acid:
It is well absorbed in the digestive tract. The maximal concentration after oral administration is 4 hours. Metabolized mainly in the liver. It is excreted by the kidneys through the intestines and sweat unchanged and as metabolites.
Indications
Indications
For children aged 6 to 15 years for short-term treatment of conditions accompanied by:
– elevated body temperature, runny nose with “colds”;
Active ingredient
Active ingredient
Composition
Composition
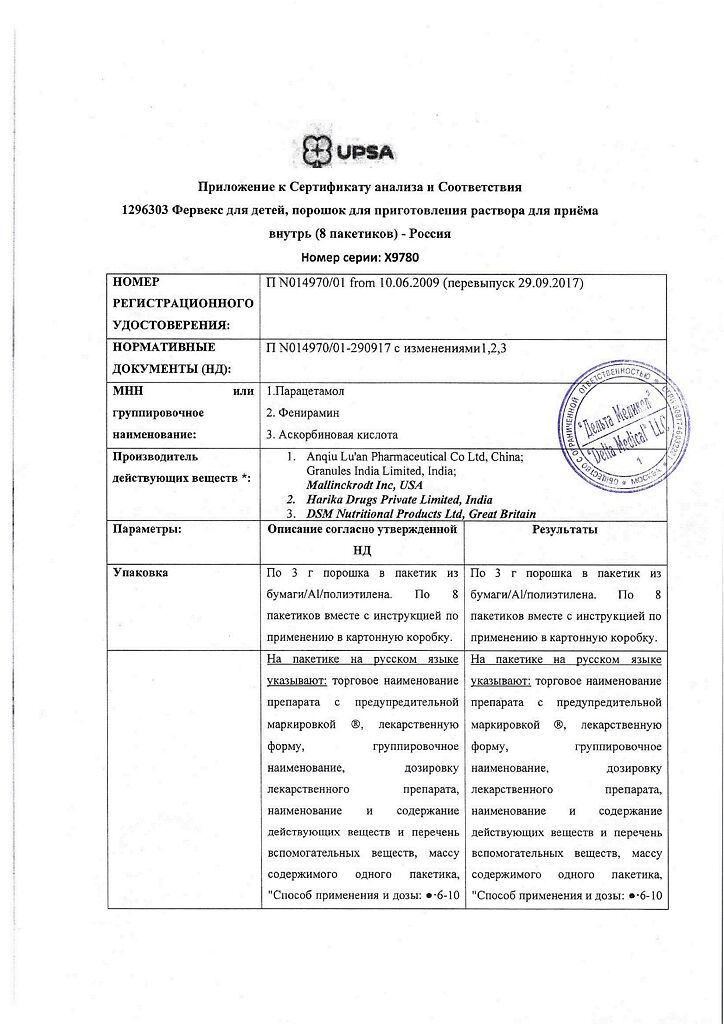
Each sachet of 3.0 g contains:
The active ingredients:
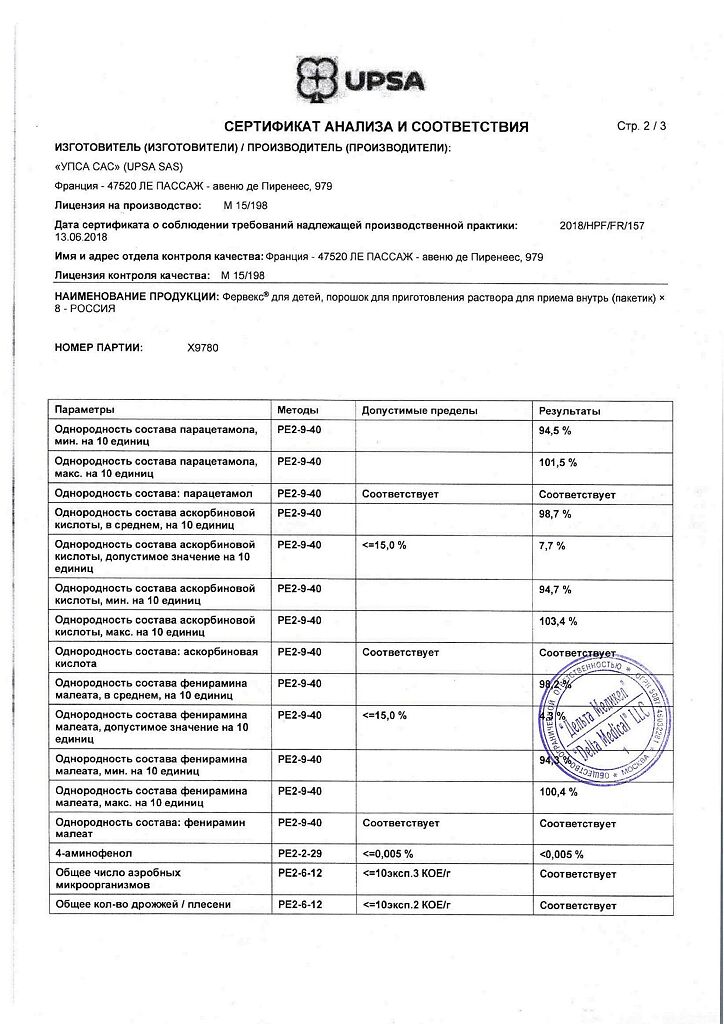
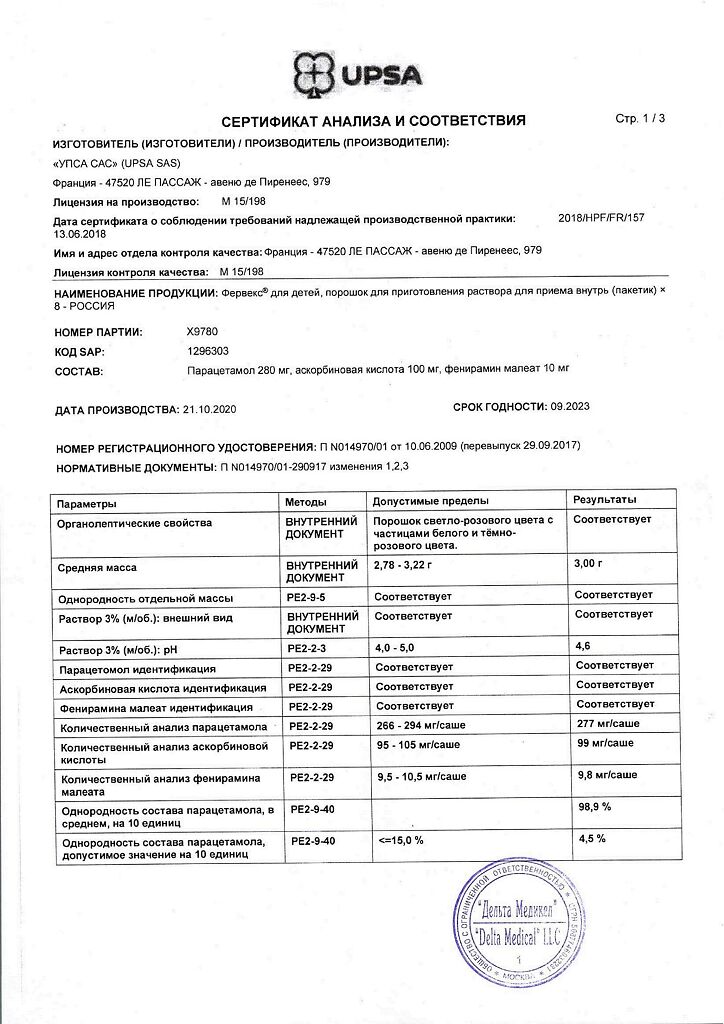
Paracetamol – 0.280 g, pheniramine maleate – 0.010 g, ascorbic acid – 0.100 g.
Auxiliary substances: mannitol – 2,260 g, citric acid – 0,030 g, povidone K30 – 0,006 g, magnesium citrate – 0,242 g, potassium acesulfame 0,036 g, raspberry flavoring * – 0,036 g.
*- Composition of raspberry flavoring: maltodextrin, E 1450 modified corn starch, E 129 dye red charming, E 133 dye diamond blue, E 110 dye sunset yellow, permastabil 505528 R1, raspberry 054428 A (ethyl acetate, isoamyl acetate, acetic acid, benzyl alcohol, triacetin, vanillin, p-hydroxy-benzylacetone), sodium chloride and/or sodium sulfate.
How to take, the dosage
How to take, the dosage
The drug is used orally. Before use, the contents of the sachet should be dissolved in a glass (200 ml) of warm water.
Depending on the age of the child, the drug is used in the following doses:
– children from 6 to 10 years – 1 sachet 2 times / day;
– children from 10 to 12 years – 1 sachet 3 times / day;
– children from 12 to 15 years – 1 sachet 4 times / day.
The interval between doses of the drug is at least 4 hours. Duration of treatment is not more than 3 days.
Interaction
Interaction
Special Instructions
Special Instructions
Contraindications
Contraindications
Side effects
Side effects
Overdose
Overdose
Pregnancy use
Pregnancy use
Similarities
Similarities
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at 15° to 25°C. |
| Manufacturer | UTSA SAS, France |
| Medication form | Powder for preparation of solution for oral administration |
| Brand | UTSA SAS |
Other forms…
Related products
Buy Fervex for children, 8 pcs. with delivery to USA, UK, Europe and over 120 other countries.