No products in the cart.
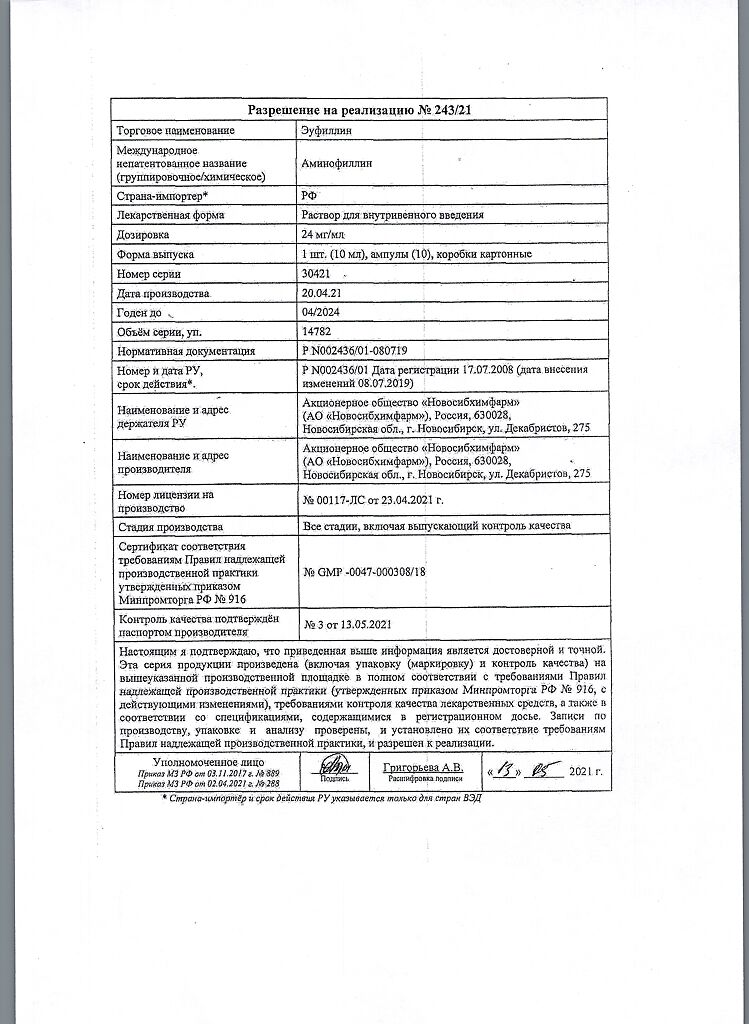
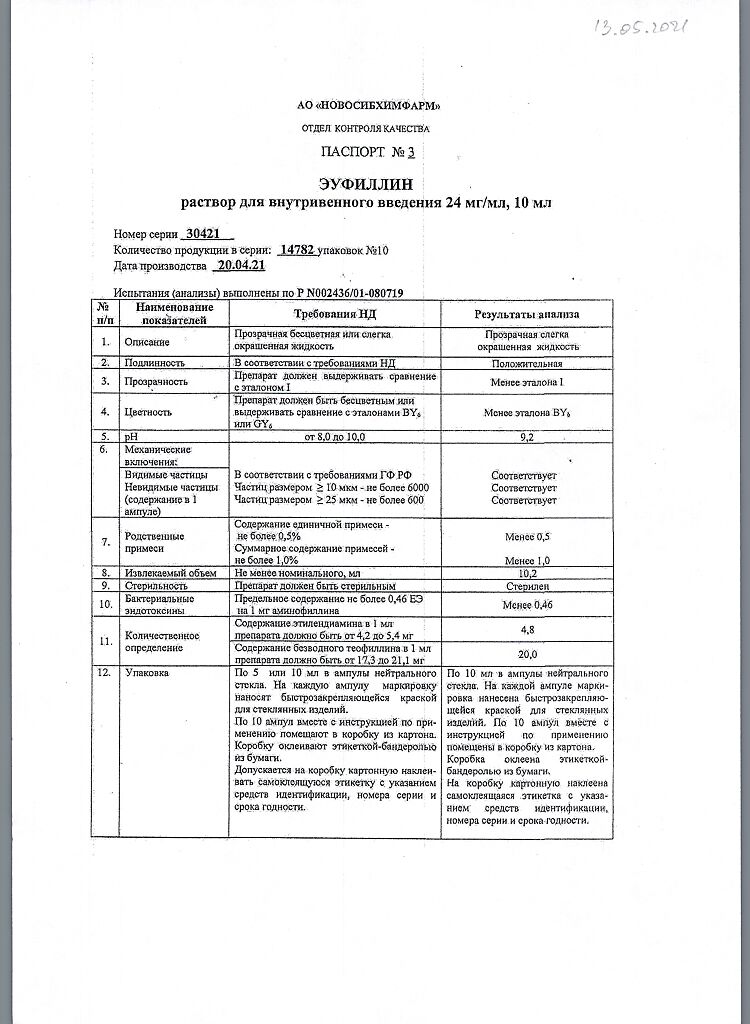
Eufillin, 24 mg/ml 10 ml 10 pcs
€4.00 €3.64
Description
Euphylline is a bronchodilator and an FDE inhibitor. It is ethylenediamine salt of theophylline (which facilitates solubility and increases absorption). It has a bronchodilator effect, apparently due to a direct relaxing effect on the smooth muscles of the airways and blood vessels of the lungs.
It is believed that this effect is caused by selective inhibition of the activity of specific FDEs, which leads to an increase in the intracellular concentration of cAMP. The results of experimental studies in vitro show that the main role seems to be played by isoenzymes of types III and IV.
The suppression of the activity of these isoenzymes may also cause some side effects of aminophylline (theophylline), including vomiting, arterial hypotension and tachycardia. It blocks adenosine (purine) receptors, which may be a factor of action on the bronchi.
It reduces airway hyperresponsiveness associated with the late phase of the response caused by inhaling allergens through an unknown mechanism that is not related to FDE inhibition or adenosine action blockade. There are reports that aminophylline increases the number and activity of T-suppressors in peripheral blood.
Increases mucociliary clearance, stimulates diaphragm contraction, improves respiratory and intercostal muscle function, stimulates the respiratory center, increases its sensitivity to carbon dioxide and improves alveolar ventilation, which ultimately leads to a reduction in the severity and frequency of apnea episodes. By normalizing respiratory function, it promotes blood oxygenation and decreases carbon dioxide concentration. It enhances lung ventilation in conditions of hypokalemia.
It has a stimulating effect on heart activity, increases strength and heart rate, increases coronary blood flow and increases myocardial oxygen demand. Reduces the tone of blood vessels (mainly vessels of the brain, skin and kidneys). It has a peripheral venodilator effect, reduces pulmonary vascular resistance, lowers the pressure in the small circle of the circulation.
increases renal blood flow, has a moderate diuretic effect. It dilates extrahepatic biliary tracts. Stabilizes membranes of mast cells, inhibits the release of mediators of allergic reactions. Inhibits platelet aggregation (inhibits platelet activation factor and PgE2α), increases resistance of red blood cells to deformation (improves rheological properties of blood), reduces thrombosis and normalizes microcirculation. It has a tocolytic effect and increases the acidity of gastric juice. In high doses it has epileptogenic effect.
Pharmacokinetics
In the body aminophylline is metabolized at physiological pH values with release of free theophylline. Bronchodilator properties are evident at plasma theophylline concentrations of 10-20 µg/ml. Concentrations above 20 mg/ml are toxic. The excitatory effect on the respiratory center is realized at a lower concentration of 5-10 µg/ml.
Theophylline’s binding to plasma proteins is approximately 40%; in neonates as well as in adults with disease, the binding decreases. Binding to plasma proteins in adults is about 60%; in newborns, 36%; in patients with cirrhosis, 36%. It penetrates the placental barrier (the concentration in the fetal serum is slightly higher than in the mother’s serum). It is excreted with the breast milk.
Theophylline is metabolized in the liver with the participation of several cytochrome P450 isoenzymes, the most important of which is CYP1A2. During metabolism 1,3-dimethylureic acid, 1-methylureic acid and 3-methylxanthine are formed. These metabolites are excreted in the urine. In adults, 10% is excreted unchanged. In infants, a significant portion is excreted as caffeine (due to the immaturity of its further metabolic pathways), unchanged – 50%.
Significant individual differences in the rate of hepatic metabolism of theophylline cause marked variability in values of clearance, plasma concentrations and elimination half-life. Such factors as age, tobacco smoking habits, diet, diseases, and concomitant drug therapy affect hepatic metabolism.
Theophylline’s T1/2 in non-smoking patients with bronchial asthma with practically no pathological changes from other organs and systems is 6-12 hours, in smokers – 4-5 hours, in children – 1-5 hours, in newborns and premature infants – 10-45 hours.
Theophylline T1/2 is increased in elderly people and in patients with heart failure or liver disease.
Clearance is decreased in heart failure, hepatic dysfunction, chronic alcoholism, pulmonary edema, and chronic obstructive pulmonary disease.
Ethylenediamine does not affect the pharmacokinetics of theophylline.
Indications
Indications
Broncho-obstructive syndrome of any origin: bronchial asthma (the drug of choice in patients with bronchial asthma of physical exertion and as an additional remedy for other forms), chronic obstructive pulmonary disease, emphysema, chronic obstructive bronchitis, hypertension in the pulmonary circulation, night apnea.
Ischemic cerebrovascular accident (as part of combination therapy to reduce intracranial pressure).
Left ventricular heart failure (as part of complex therapy).
Pharmacological effect
Pharmacological effect
Eufillin is a bronchodilator, a PDE inhibitor. It is the ethylenediamine salt of theophylline (which facilitates solubility and increases absorption). It has a bronchodilator effect, apparently due to a direct relaxing effect on the smooth muscles of the respiratory tract and blood vessels of the lungs.
It is believed that this effect is caused by selective inhibition of the activity of specific PDEs, which leads to an increase in the intracellular concentration of cAMP. The results of in vitro experimental studies show that the main role appears to be played by type III and IV isoenzymes.
Suppression of the activity of these isoenzymes may also cause some side effects of aminophylline (theophylline), including. vomiting, arterial hypotension and tachycardia. Blocks adenosine (purine) receptors, which may be one of the factors affecting the bronchi.
Reduces airway hyperresponsiveness associated with the late phase response caused by inhaled allergens through an unknown mechanism that is not due to PDE inhibition or blockade of adenosine. There are reports that aminophylline increases the number and activity of T-suppressor cells in the peripheral blood.
Increases mucociliary clearance, stimulates contraction of the diaphragm, improves the function of the respiratory and intercostal muscles, stimulates the respiratory center, increases its sensitivity to carbon dioxide and improves alveolar ventilation, which ultimately leads to a decrease in the severity and frequency of apnea episodes. By normalizing respiratory function, it helps saturate the blood with oxygen and reduce the concentration of carbon dioxide. Strengthens ventilation of the lungs in conditions of hypokalemia.
It has a stimulating effect on the activity of the heart, increases strength and heart rate, increases coronary blood flow and increases the myocardial oxygen demand. Reduces the tone of blood vessels (mainly those of the brain, skin and kidneys). It has a peripheral venodilating effect, reduces pulmonary vascular resistance, and lowers pressure in the pulmonary circulation.
Increases renal blood flow and has a moderate diuretic effect. Expands extrahepatic bile ducts. Stabilizes mast cell membranes, inhibits the release of mediators of allergic reactions. Inhibits platelet aggregation (suppresses platelet activating factor and PgE2α), increases the resistance of red blood cells to deformation (improves the rheological properties of blood), reduces thrombus formation and normalizes microcirculation. It has a tocolytic effect, increases the acidity of gastric juice. In high doses it has an epileptogenic effect.
Pharmacokinetics
In the body, aminophylline is metabolized at physiological pH values to release free theophylline. Bronchodilating properties appear at plasma theophylline concentrations of 10-20 mcg/ml. Concentrations above 20 mg/ml are toxic. The stimulating effect on the respiratory center is realized at a lower concentration – 5-10 μg/ml.
Theophylline binding to plasma proteins is approximately 40%; in newborns, as well as in adults with diseases, binding decreases. Plasma protein binding in adults is about 60%, in newborns – 36%, in patients with liver cirrhosis – 36%. Penetrates the placental barrier (the concentration in the fetal blood serum is slightly higher than in the maternal serum). Excreted in breast milk.
Theophylline is metabolized in the liver with the participation of several cytochrome P450 isoenzymes, the most important of which is CYP1A2. During metabolism, 1,3-dimethyluric acid, 1-methyluric acid and 3-methylxanthine are formed. These metabolites are excreted in the urine. 10% is excreted unchanged in adults. In newborns, a significant portion is excreted in the form of caffeine (due to the immaturity of the pathways for its further metabolism), unchanged – 50%.
Significant individual differences in the rate of hepatic metabolism of theophylline are the cause of pronounced variability in clearance values, plasma concentrations, and half-life. Hepatic metabolism is influenced by factors such as age, addiction to tobacco smoking, diet, diseases, and concurrent drug therapy.
T1/2 of theophylline in non-smoking patients with bronchial asthma with virtually no pathological changes in other organs and systems is 6-12 hours, in smokers – 4-5 hours, in children – 1-5 hours, in newborns and premature babies – 10-45 hours.
T1/2 of theophylline increases in the elderly and in patients with heart failure or liver disease.
Clearance decreases with heart failure, liver dysfunction, chronic alcoholism, pulmonary edema, chronic obstructive pulmonary disease.
Ethylenediamine does not affect the pharmacokinetics of theophylline.
Special instructions
Special instructions
Use caution when consuming large amounts of caffeine-containing foods or drinks during treatment.
Before use, the drug solution must be warmed to body temperature.
Elderly patients are advised to reduce the dose of the drug due to its slow elimination from the body.
Smoking patients are advised to increase the dose due to the accelerated elimination of the drug from the body.
Impact on the ability to drive vehicles, operate machinery and engage in other activities that require concentration and speed of psychomotor reactions.
During treatment with the drug, it is not recommended to drive vehicles, machinery, or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Aminophylline
Composition
Composition
1 ml contains:
Active substance:
aminophylline for injection (aminophylline) – 24.0 mg;
Excipient:
water for injection – up to 1 ml
Pregnancy
Pregnancy
If it is necessary to use the drug during pregnancy, the expected benefit to the mother should be compared with the potential risk to the fetus.
If it is necessary to use the drug during breastfeeding, breastfeeding should be discontinued.
Contraindications
Contraindications
Hypersensitivity to the drug, as well as to xanthine derivatives: caffeine, pentoxifylline, theobromine.
Severe arterial hypotension or hypertension, paroxysmal tachycardia, extrasystole, myocardial infarction with cardiac arrhythmias, epilepsy, increased convulsive readiness, hypertrophic obstructive cardiomyopathy, thyrotoxicosis, pulmonary edema, severe coronary insufficiency, liver and/or renal failure, hemorrhagic stroke, retinal hemorrhage, bleeding in recent history.
With caution
Sepsis, peptic ulcer of the stomach and duodenum (history), old age (over 55 years), uncontrolled hypothyroidism (possibility of cumulation), widespread vascular atherosclerosis, prostatic hyperplasia, children under 14 years of age (due to possible side effects).
Side Effects
Side Effects
From the nervous system: dizziness, headache, insomnia, agitation, anxiety, irritability, tremor.
From the cardiovascular system: palpitations, tachycardia (including in the fetus when taken by a pregnant woman in the third trimester), arrhythmias, decreased blood pressure, cardialgia, increased frequency of angina attacks.
From the digestive system: gastralgia, nausea, vomiting, gastroesophageal reflux, heartburn, exacerbation of peptic ulcer, diarrhea, with long-term use – decreased appetite.
Allergic reactions: skin rash, skin itching, exfoliative dermatitis, fever.
Other: chest pain, tachypnea, feeling of “hot flashes” in the face, albuminuria, hematuria, hypoglycemia, increased diuresis, increased sweating.
Side effects decrease when the dose of the drug is reduced, when the method of administration is changed (from jet to drip).
Local reactions: compaction, hyperemia, pain at the injection site.
Interaction
Interaction
Pharmaceutically incompatible with acid solutions.
Increases the likelihood of developing side effects of glucocorticosteroids, mineralocorticosteroids (hypernatremia), drugs for general anesthesia (increases the risk of ventricular arrhythmias), drugs that excite the central nervous system (increases neurotoxicity).
Antidiarrheal drugs and oral estrogen-containing contraceptives weaken the effect of aminophylline (they bind to the cytochrome P450 enzymatic system and change the metabolism of aminophylline).
Rifampicin, phenobarbital, phenytoin, isoniazid, carbamazepine and moracizine, being inducers of microsomal oxidation, increase the clearance of aminophylline, which may require an increase in its dose.
When used simultaneously with macrolide antibiotics, lincomycin, allopurinol, cimetidine, isoprenaline, small doses of ethanol, disulfiram, fluoroquinolones, recombinant interferon alpha, methotrexate, mexiletine, propafenone, thiabendazole, ticlopidine, verapamil and with influenza vaccination, the intensity of action of aminophylline may increase, which may require a reduction in its dose.
Enhances the effect of beta-adrenergic stimulants and diuretics (including by increasing glomerular filtration), reduces the effectiveness of lithium preparations and beta-blockers.
Compatible with antispasmodics, do not use in combination with other xanthine derivatives.
Prescribe with caution simultaneously with anticoagulants.
Overdose
Overdose
Symptoms: loss of appetite, gastralgia, diarrhea, nausea, vomiting (including blood). gastrointestinal bleeding, tachypnea, facial skin flushing, tachycardia, ventricular arrhythmias, insomnia, motor agitation, anxiety, photophobia. tremor, convulsions.
In severe poisoning, epileptoid convulsions may develop (especially in children without any warning signs), hypoxia, metabolic acidosis, hyperglycemia, hypokalemia, decreased blood pressure, skeletal muscle necrosis, confusion, renal failure with myoglobinuria.
Treatment: drug withdrawal, forced diuresis, hemosorption, plasma sorption, hemodialysis (low efficiency, peritoneal dialysis is ineffective), symptomatic therapy (including intravenous metoclopramide for vomiting). If convulsions occur, maintain airway patency and administer oxygen therapy. To relieve seizures, administer diazepam 0.1-0.3 mg/kg intravenously (but not more than 10 mg).
Storage conditions
Storage conditions
In a place protected from light at a temperature of 10 to 25C. Freezing is unacceptable.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Novosibkhimpharm, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature of 10 to 25C. Freezing is unacceptable. |
| Manufacturer | Novosibkhimpharm, Russia |
| Medication form | solution |
| Brand | Novosibkhimpharm |
Other forms…
Related products
Buy Eufillin, 24 mg/ml 10 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.