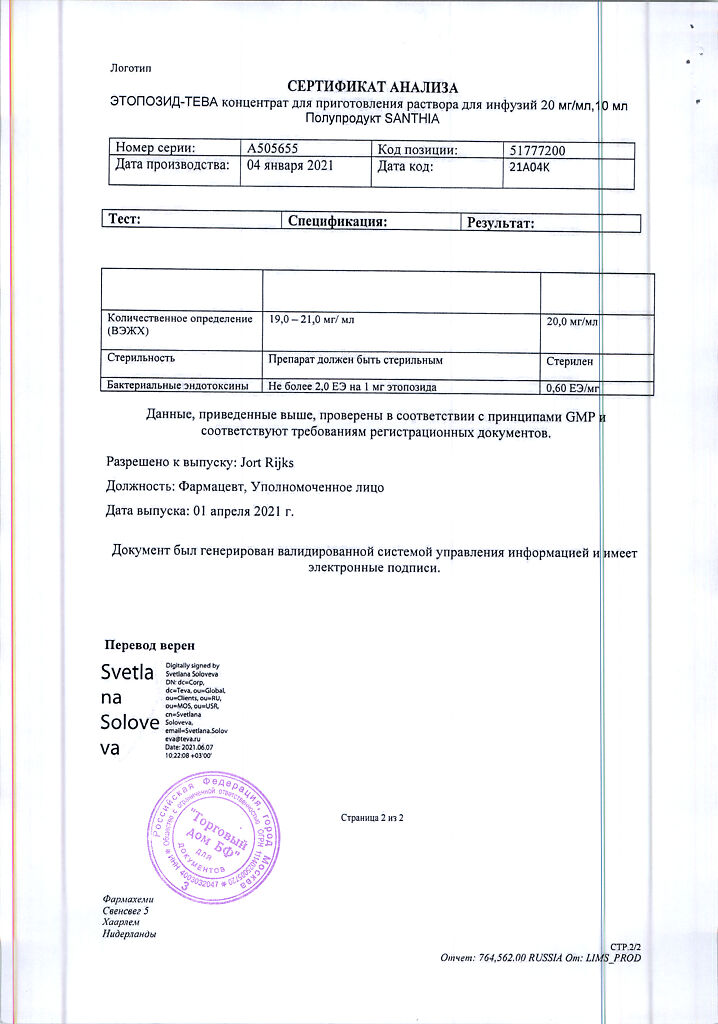
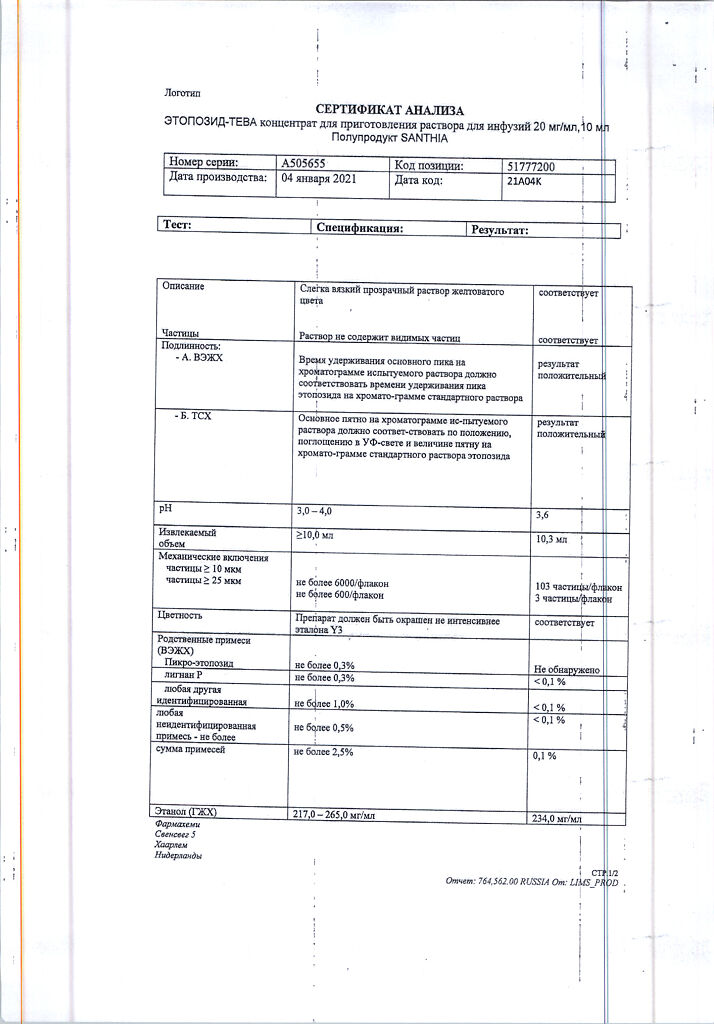
No products in the cart.
Description
Pharmacotherapeutic group:antitumor agent, alkaloid.
ATX code: L01CB01
Pharmacological properties
Pharmacodynamics
The etoposide is a semi-synthetic derivative of podophyllotoxin. The mechanism of action is associated with inhibition of topoisomerase II. Etoposide has cytotoxic effect due to DNA damage. The drug blocks mitosis, causing cell death in the G2 phase and the late S phase of the mitotic cycle. High concentrations of the drug cause cell lysis in the premitotic phase. Etoposide also inhibits nucleotide penetration through the plasma membrane, which prevents DNA synthesis and repair.
Pharmacokinetics
. After intravenous administration, the maximum plasma concentration (Cmax) is 30 µg/mL and is reached after 1-2 hours. The drug is detected in pleural fluid, saliva, liver tissue, spleen, kidneys, brain tissue. Values of etoposide concentration in cerebrospinal fluid vary from undetectable values to 5% of blood plasma concentration. Etoposide penetrates through the blood-brain and placental barriers. Binding with blood plasma proteins is about 97%. Total clearance of the drug is 16-36 ml/min/m2.
Atoposide is actively metabolized in the body. Excretion occurs in a biphasic manner. In adults with normal renal and hepatic function, the elimination half-life (T1/2) averages 0.6-2 hours in the initial phase and 5.3-11 hours in the terminal phase. It is excreted by the kidneys – 40-60% unchanged and 15% as metabolites within 48-72 hours. 2-16% is excreted by the intestine within 72 hours.
Indications
Indications
Germ cell tumors of the testicle and ovaries;
small cell lung cancer;
lymphogranulomatosis;
non-Hodgkin’s lymphoma;
acute monoblastic and myeloblastic leukemia;
Ewing’s sarcoma;
trophoblastic tumors;
stomach cancer, Kaposi’s sarcoma and neuroblastoma.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antitumor agent, alkaloid.
ATX code: L01СB01
Pharmacological properties
Pharmacodynamics
Etoposide is a semi-synthetic derivative of podophyllotoxin. The mechanism of action is associated with inhibition of topoisomerase II. Etoposide has a cytotoxic effect due to DNA damage. The drug blocks mitosis, causing cell death in the G2 phase and late S phase of the mitotic cycle. High concentrations of the drug cause cell lysis in the premitotic phase. Etoposide also inhibits the passage of nucleotides through the plasma membrane, which interferes with DNA synthesis and repair.
Pharmacokinetics
After intravenous administration, the maximum plasma concentration (Cmax) is 30 mcg/ml and is achieved within 1-2 hours. The drug is found in pleural fluid, saliva, liver tissue, spleen, kidneys, and brain tissue. Concentrations of etoposide in cerebrospinal fluid vary from undetectable values to 5% of plasma concentrations. Etoposide penetrates the blood-brain and placental barriers. The binding to plasma proteins is about 97%. The total clearance of the drug is 16-36 ml/min/m2.
Etoposide is actively metabolized in the body. Isolation is carried out in a two-phase manner. In adults with normal renal and liver function, the half-life (T1/2) in the initial phase averages 0.6-2 hours and in the terminal phase – 5.3-11 hours. Excreted by the kidneys – 40-60% unchanged and 15% in the form of metabolites within 48-72 hours. 2-16% is excreted by the intestines within 72 hours.
Special instructions
Special instructions
Etoposide-Teva should only be used under the constant supervision of a physician experienced in the treatment of cytotoxic drugs. Before using the drug, the doctor must assess the benefit/risk ratio for each patient and the risk of side effects. Most adverse reactions are reversible if detected early. If severe reactions occur, reduce the dose of the drug or discontinue its use and initiate appropriate treatment. Resumption of drug therapy should be done with caution and under close medical supervision for possible recurrence of toxicity.
When working with Etoposide-Teva, you should follow the rules for handling cytotoxic drugs. In case of contact with skin or mucous membrane, the affected areas should be washed immediately with soap and water.
Myelosuppression
When using the drug, it is possible to develop severe myelosuppression (sometimes with death) and, as a result, infection or bleeding.
Suppression of bone marrow function is the dose-limiting effect of Etoposide-Teva. It is necessary to conduct a regular detailed clinical blood test before starting treatment, during breaks and before each subsequent course of the drug. If radiation therapy and/or chemotherapy were administered before starting therapy with Etoposide-Teva, a sufficient interval should be observed between these two types of treatment to ensure restoration of bone marrow function. The choice of dosage regimen should be based on the severity of bone marrow suppression.
If the platelet count decreases below 100,000 cells/μl and/or the absolute neutrophil count below 1500 cells/μl (with the exception of myelosuppression caused by cancer), etoposide therapy should be discontinued until blood counts are completely restored.
In the event of the development of severe hematological toxicity (a decrease in the number of neutrophils less than 500/mm3 for more than 5 days or in combination with fever/infection, a decrease in the number of platelets less than 25,000/mm3), other manifestations of grade III and IV toxicity, in the case of a decrease in CC <50 ml/min, further doses of the drug should be adjusted.
Secondary leukemia
The occurrence of acute leukemia, which may occur with or without myelodysplastic syndrome, has been described in patients treated with etoposide chemotherapy. To date, neither the cumulative risk nor the predisposing factors associated with the development of secondary leukemia are known. Cumulative etoposide dose and chemotherapy regimens have been proposed as risk factors but have not been clearly defined. In patients with incident secondary leukemia who received epipodophyllotoxins, 11q23 chromosomal abnormalities were identified. These chromosomal abnormalities have also been identified in patients with secondary leukemia after chemotherapy that does not contain epipodophyllotoxins, and in de novo leukemia. The average period for the onset of leukemia after chemotherapy is about 32 months.
Hypersensitivity
An anaphylactic reaction is possible (chills, fever, tachycardia, bronchospasm, shortness of breath, arterial hypotension), which can be fatal.
If anaphylactic reactions occur, use of the drug Etoposide-Teva should be stopped and treatment with vasopressor drugs, glucocorticosteroids and/or antihistamines should be started.
Arterial hypotension
Etoposide should be administered intravenously slowly (usually over 30 to 60 minutes), since rapid administration may cause severe hypotension.
Low serum albumin concentration
Patients with low serum albumin concentrations are at increased risk of etoposide-related toxicity.
Reactions at the injection site
Caution should be exercised regarding possible extravasation of the drug, since it has a pronounced local irritant effect and in some cases can lead to necrosis of surrounding tissues.
If signs of extravasation (burning sensation) appear, infusion with Etoposide-Teva should be stopped immediately. The remaining drug is injected into another vein. Subcutaneous injections of hydrocortisone are made around the affected area and 1% hydrocortisone ointment is applied under a dry bandage (until the skin hyperemia disappears – usually for 24 hours).
Impaired kidney and liver function
Caution must be exercised when using the drug in patients with mild to moderate impairment of liver and kidney function; correction of the dosage regimen and regular monitoring of liver and kidney function are necessary.
Tumor lysis syndrome
Tumor lysis syndrome (sometimes fatal) has been reported when etoposide is used in combination with other chemotherapy drugs.
Effect on fertility
Men and women of childbearing age should use reliable methods of contraception during treatment with Etoposide-Teva and for at least 6 months after the end of treatment. Because the drug may reduce fertility in men, sperm preservation should be considered before starting treatment.
Ethanol
The intravenous solution of Etoposide-Teva contains ethyl alcohol as an excipient, which may be a risk factor for patients suffering from liver disease, alcoholism and epilepsy, as well as for children. The drug contains polysorbate-80. In premature infants, the use of injectable vitamin E containing polysorbate 80 has been reported to result in liver and kidney failure, pulmonary dysfunction, thrombocytopenia, and ascites.
Impact on the ability to drive vehicles and machinery
Due to the likelihood of side effects such as dizziness, weakness, drowsiness, nausea, vomiting, transient cortical blindness, arterial hypotension, and also given the ethanol content in the drug, you should refrain from engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Etoposide
Composition
Composition
1 ml of solution contains:
active ingredient: etoposide 20 mg;
excipients: citric acid 2 mg, polysorbate-80 80 mg, ethanol (absolute ethanol) 241 mg, macrogol-300 to 1 ml (from 670 to 700 mg).
Pregnancy
Pregnancy
Women with preserved childbearing potential/contraception in men and women
Women of childbearing potential should use appropriate methods of contraception to avoid pregnancy while using etoposide. Men and women of childbearing age should use reliable methods of contraception during treatment with Etoposide-Teva and for at least 6 months after the end of treatment.
Pregnancy
There are no controlled studies of the use of etoposide in pregnant women. Animal studies have shown embryotoxic, teratogenic and mutagenic effects of etoposide. Therefore, pregnant women should not prescribe the drug.
Breast-feeding
Etoposide passes into breast milk.
Therefore, the use of the drug during breastfeeding is contraindicated, or in order to avoid the toxic effect of the drug on the baby, breastfeeding should be stopped during the treatment period.
Fertility
Because the drug may reduce fertility in men, sperm preservation should be considered before starting treatment.
Contraindications
Contraindications
Hypersensitivity to etoposide or excipients included in the drug;
simultaneous use with live vaccines, including yellow fever vaccine in patients with myelosuppression;
severe myelosuppression (neutrophil count below 1500/μl and/or platelet count below 100,000/μl);
severe liver and kidney dysfunction;
acute infections;
pregnancy and lactation period.
With caution: mild to moderate dysfunction of the liver and kidneys, brain diseases, alcoholism, epilepsy, childhood (due to the presence of polysorbate-80 and ethanol in the drug).
Side Effects
Side Effects
The incidence of adverse reactions is estimated as follows: very often (≥1/10), often (from ≥1/100 to ˂1/10), infrequently (from ≥1/1000 to ˂1/100), rarely (from ≥1/10000 to ˂1/1000), very rarely (˂1/10000), frequency unknown (cannot be estimated by available data).
Infections and infestations: often – infections.
Benign and malignant neoplasms: often – acute leukemia; frequency unknown – acute promyelocytic leukemia.
From the blood and lymphatic system: very often – anemia, leukopenia, neutropenia, thrombocytopenia, myelosuppression (with possible death).
From the immune system: often – anaphylactic reactions; frequency unknown – angioedema, bronchospasm.
From the side of metabolism: frequency unknown – tumor lysis syndrome; rarely – metabolic acidosis, hyperuricemia.
From the nervous system: often – dizziness; infrequently – peripheral neuropathy; rarely – transient cortical blindness, neurotoxicity (including drowsiness and fatigue), optic neuritis, convulsions.
From the heart: often – arrhythmia, myocardial infarction.
From the side of blood vessels: often – increased blood pressure, transient decrease in blood pressure due to rapid intravenous administration of the drug; infrequently – hemorrhages; rarely – flushes of blood to the face.
From the respiratory system: rarely – interstitial pneumonitis, laryngospasm, cyanosis, apnea, pulmonary fibrosis; frequency unknown – bronchospasm.
From the digestive system: very often – abdominal pain, anorexia, constipation, nausea, vomiting; often – diarrhea, mucositis (including stomatitis and esophagitis); rarely – dysphagia (difficulty swallowing), dysgeusia (taste perversion).
From the liver and biliary tract: very often – increased activity of liver transaminases, bilirubin, hepatotoxicity.
From the skin and subcutaneous tissues: very often – alopecia, pigmentation; often – rash, itching, urticaria; rarely – Stevens-Johnson syndrome, toxic epidermal necrolysis, relapse of radiation dermatitis.
From the genitourinary system: frequency unknown – decreased fertility.
General disorders: very often – asthenia, weakness; often – phlebitis, extravasation; rarely – fever.
Interaction
Interaction
Etoposide should not be mixed with other drugs in the same solution.
Pharmaceutically incompatible with solutions with alkaline pH values.
Etoposide can potentiate the cytotoxic and myelosuppressive effects of other drugs (for example, cyclosporine), radiation therapy.
The antitumor effect of etoposide is enhanced when used in combination with cisplatin (it should be taken into account that in patients previously treated with cisplatin, the elimination of etoposide may be impaired).
When used simultaneously with cisplatin, the clearance of etoposide is reduced.
When used simultaneously with phenytoin, the clearance of etoposide increases and its effectiveness decreases.
Etoposide may reduce the effectiveness of antiepileptic drugs when used concomitantly.
Etoposide enhances the effect of indirect anticoagulants (the International Normalized Ratio (INR) increases).
Phenylbutazone, sodium salicylate and salicylic acid may affect the binding of etoposide to plasma proteins. Cross-resistance may develop between anthracyclines and etoposide.
Due to the immunosuppressive effect of the drug and the possibility of severe infection, live vaccines should not be used during chemotherapy (see section “Contraindications”). The use of the yellow fever vaccine increases the risk of post-vaccination complications of a systemic nature with a fatal outcome. Vaccination should be carried out 3 months after completion of therapy.
The occurrence of acute leukemia has been reported in patients receiving etoposide in combination with other anticancer drugs (bleomycin, cisplatin, ifosfamide, methotrexate).
Overdose
Overdose
Intravenous administration of the drug at a total dose of 2.4-3.5 g/m2 for 3 days led to the development of severe mucositis and myelotoxicity. The development of metabolic acidosis and hepatotoxicity has also been reported when using doses of the drug higher than recommended.
Treatment: symptomatic therapy is carried out. A specific antidote is not known. Monitoring of vital body functions is necessary.
Storage conditions
Storage conditions
Store at a temperature of 15 to 25 °C, protected from light.
Keep out of the reach of children!
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Farmahemi B.V., Netherlands
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at 15 to 25 oC, protected from light. Keep out of reach of children! |
| Manufacturer | Pharmahemi B.V., The Netherlands |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Pharmahemi B.V. |
Related products
Buy Etoposide-Teva, 20 mg/ml 10 ml with delivery to USA, UK, Europe and over 120 other countries.