Subtotal: €22.00
Oestrogel, transdermal gel 600 mcg/g 80 g
€60.50 €53.09
The active ingredient of the drug EstroGel® – 17β-estradiol is chemically and biologically identical to endogenous human estradiol.
It has estrogenic effects on the main target organs: ovaries, endometrium, vaginal epithelium, mammary glands, urethra, hypothalamus, pituitary gland, liver – similar to the effects of endogenous estrogens in the follicular phase of the menstrual cycle.
Maintains estrogen deficiency in women during menopause and reduces the severity of menopausal disorders including hot flashes, excessive sweating at night, atrophic changes in the urinary tract (atrophic vulvovaginitis, dyspareunia, urinary incontinence) and psycho-emotional disorders.
The clinical effectiveness of Estrogel® in treating menopausal symptoms is comparable to that of oral estrogen.
Estradiol helps reduce total cholesterol concentrations without changing the cholesterol/HDL ratio.
It has a procoagulant effect, increases the synthesis in the liver of vitamin K-dependent clotting factors (II, VII, IX, X), reduces the concentration of antithrombin III.
Estradiol prevents loss of bone mass associated with natural menopause or ovariectomy.
Estrogen deficiency in postmenopause is associated with decreased bone mineral density (BMD). The effect of estrogen on BMDM is dose-dependent and appears to continue for as long as hormone replacement therapy (HRT) is in place. After discontinuation of HRT, BMSCs begin to decline at the same rate as they did before the start of HRT.
The data from the randomized, placebo-controlled Women’s Health Initiative (WHI) study and a meta-analysis of clinical trials showed that MHT with estrogen alone or estrogen in combination with gestagens in healthy postmenopausal women reduces the risk of hip, spine, and other osteoporotic fractures. There is also limited evidence that MHT can prevent bone fractures in women with low BMD and/or established osteoporosis.
Indications
– hormone replacement therapy for symptoms of estrogen deficiency, treatment of menopausal syndrome associated with natural or surgical menopause;
– prevention of osteoporosis during the postmenopausal period in women at high risk of fractures with intolerance or contraindications to the use of other drugs for the prevention of osteoporosis.
Pharmacological effect
The active ingredient of the drug Estrogel® – 17β-estradiol is chemically and biologically identical to endogenous human estradiol.
It has an estrogenic effect on the main target organs: ovaries, endometrium, vaginal epithelium, mammary glands, urethra, hypothalamus, pituitary gland, liver – similar to the action of endogenous estrogens in the follicular phase of the menstrual cycle.
Replenishes estrogen deficiency in women during menopause and reduces the severity of menopausal disorders, including hot flashes, increased sweating at night, atrophic changes in the genitourinary tract (atrophic vulvovaginitis, dyspareunia, urinary incontinence), psycho-emotional disorders.
The clinical effectiveness of Estrogel® in the treatment of menopausal symptoms is comparable to that of oral estrogens.
Estradiol helps reduce the concentration of total cholesterol without changing the cholesterol/HDL ratio.
It has a procoagulant effect, increases the synthesis of vitamin K-dependent blood coagulation factors (II, VII, IX, X) in the liver, reduces the concentration of antithrombin III.
Estradiol prevents bone loss associated with natural menopause or oophorectomy.
Estrogen deficiency during postmenopause is associated with a decrease in bone mineral density (BMD). The effect of estrogen on BMD is dose-dependent and appears to last as long as hormone replacement therapy (HRT) is administered. After stopping HRT, BMD begins to decrease at the same rate as before it began.
Data from the randomized, placebo-controlled Women’s Health Initiative (WHI) trial and a meta-analysis of clinical trials showed that HRT with estrogens alone or with estrogens plus progestins in healthy postmenopausal women reduces the risk of hip, vertebral and other osteoporotic fractures. There is also limited evidence that HRT can prevent bone fractures in women with low BMD and/or established osteoporosis.
Special instructions
When treating postmenopausal symptoms, HRT should only be started if symptoms are present that adversely affect quality of life. A detailed assessment of the risks and benefits should be carried out at least once a year and HRT should be prescribed only if the benefits outweigh the risks.
Data regarding the risks associated with HRT for the treatment of premature menopause are limited. However, given the low absolute risk of HRT in younger women, the benefit-risk ratio may be more favorable in these women than in older women.
Before starting or re-prescribing HRT, it is necessary to obtain a complete personal and family history. A medical examination should be conducted to identify possible contraindications and observe the necessary precautions when taking the drug (including examination of the pelvic organs and mammary glands). During treatment, periodic examinations are recommended. The frequency and methods included in it are determined for each specific case individually. Tests, including mammography, should be carried out in accordance with accepted standards and adapted to the individual clinical needs of each individual case.
When a patient is using HRT medications, it is necessary to carefully evaluate all the benefits and risks of therapy.
Conditions that require monitoring
If any of the following conditions are present, previously encountered and/or aggravated during pregnancy or previous hormonal therapy, the patient should be under constant medical supervision. It should be taken into account that these conditions may, in rare cases, recur or worsen during treatment with Estrogel®, in particular:
uterine fibroids or endometriosis;
risk factors for the development of thromboembolic diseases;
risk factors for estrogen-dependent tumors (presence of first-degree relatives with breast cancer);
arterial hypertension;
liver diseases (for example, liver adenoma);
diabetes mellitus with or without diabetic angiopathy;
cholelithiasis;
migraine and/or severe headache;
systemic lupus erythematosus;
history of endometrial hyperplasia;
epilepsy;
bronchial asthma;
otosclerosis;
hereditary angioedema.
Reasons for immediate discontinuation of therapy
Therapy should be discontinued if contraindications are found and/or in the following situations:
jaundice or worsening liver function;
pronounced increase in blood pressure;
new attacks of migraine-like headache;
pregnancy.
Hyperplasia and endometrial cancer
In women with an intact uterus, the risk of endometrial hyperplasia and cancer increases when taking estrogens for a long time. The risk of developing endometrial cancer in women using estrogen only is reported to be 2- to 12-fold greater than in women not using estrogen, depending on the duration of treatment and dose of estrogen. After stopping treatment, the increased risk may persist for at least 10 years.
The addition of a progestin in the last 12 days of the month/28 days of the cycle or continuous combined estrogen-progestin therapy in women with a non-removed uterus reduces the increased risk of endometrial hyperplasia and cancer associated with estrogen-only HRT.
During the first months of treatment, breakthrough bleeding and spotting may occur. If breakthrough bleeding or spotting appears after a period of treatment or continues after discontinuation of treatment, it is necessary to conduct an examination to identify the causes of their occurrence, including an endometrial biopsy to exclude endometrial malignancy.
The use of drugs for HRT containing only estrogen can lead to precancerous or malignant transformation of residual foci of endometriosis. Therefore, in women who have undergone hysterectomy for endometriosis, consideration should be given to adding a progestin to estrogen replacement therapy for the prevention of endometrial cancer if they are known to have residual endometriosis.
Breast cancer
Available data suggest an increased risk of breast cancer in women receiving combined estrogen-progestin drugs and possibly also estrogen-only HRT drugs; this risk depends on the duration of HRT use.
The use of HRT drugs containing only estrogen
The WHI study found no increased risk of breast cancer in women who had a hysterectomy and used estrogen-only HRT.
Observational studies in most cases report a small increase in the risk of diagnosing breast cancer, which is significantly lower than in women using combined estrogen-progestin drugs.
The use of combined estrogen-progestin drugs for HRT
The WHI study and epidemiological studies provide concordant evidence of an increased risk of breast cancer in women receiving combined estrogen-progestin drugs for HRT; an increase in risk was detected after approximately 3 years of treatment.
The additional risk begins to appear after several years of treatment, but returns to baseline levels within a few (no more than 5) years after stopping treatment.
HRT, in particular combined estrogen-progestin drugs, leads to increased density of mammographic images, which may interfere with radiological detection of breast cancer.
Ovarian cancer
Ovarian cancer is much less common than breast cancer. Long-term (at least 5-10 years) use of estrogen-only HRT drugs is associated with a slight increase in the risk of developing ovarian cancer. Some studies, including the WH1 study, have found that long-term use of combined HRT drugs may carry a similar or less significant risk.
Venous thromboembolism
In women receiving HRT, there is an increase in the risk of developing VTE, in particular, deep vein thrombosis or pulmonary embolism, compared with women who did not receive HRT, by 1.3-3 times. The likelihood of developing VTE is higher in the first year of HRT than in subsequent years.
Patients with known thrombophilic disorders may have an increased risk of developing VTE, and HRT may further increase this risk. Therefore, HRT is contraindicated in such patients.
The main risk factors for the development of VTE: individual or family history, estrogen use, old age, major surgery, prolonged immobilization, severe obesity (BMI more than 30 kg/m2), pregnancy and the postpartum period, systemic lupus erythematosus, malignant neoplasms.
There is no consensus on the possible role of varicose veins in the development of VTE.
The risk of VTE increases with prolonged immobilization, major trauma, or major surgery. Taking HRT medications should be stopped 4-6 weeks before planned abdominal surgery or orthopedic surgery on the lower extremities. Treatment can be resumed after complete restoration of motor ability.
In women who do not have a history of VTE but have first-degree relatives who experienced thrombosis at a young age, screening can be offered after a detailed discussion of its limitations (screening detects only some thrombophilic disorders).
If the patient has a thrombophilic disorder with a history of thrombosis in family members, or severe defects (such as antithrombin, protein S, or protein C deficiency, or a combination of defects), HRT is contraindicated.
In women already receiving chronic anticoagulant treatment, a careful assessment of the benefit-risk ratio of HRT is required.
If VTE develops after starting treatment, the drug should be discontinued. Patients should be instructed to contact their physician immediately if potential symptoms of thromboembolism (tenderness and/or swelling of the lower extremity, sudden chest pain, shortness of breath) occur.
Coronary heart disease
Randomized controlled trials have not provided data on the preventive effect against myocardial infarction in women with or without coronary artery disease who received HRT with combined estrogen-progestin drugs or estrogen alone.
The use of HRT drugs containing only estrogen
There is no evidence from randomized controlled trials of an increased risk of coronary artery disease in patients who have undergone hysterectomy and are receiving estrogen-only HRT medications.
The use of combined estrogen-progestin drugs for HRT
When using combined estrogen-progestin drugs for HRT, there is a slight increase in the relative risk of coronary artery disease. Since the initial absolute risk of CAD is largely dependent on age, the number of additional cases of CAD due to the use of estrogens in combination with progestins in healthy women approaching menopause is extremely small, but increases with age.
Ischemic stroke
HRT with combined estrogen-progestin drugs and estrogen alone is associated with an almost 1.5-fold increase in the risk of ischemic stroke. The relative risk does not change with age or with time since menopause. However, since baseline risk of stroke is largely dependent on age, the overall risk of stroke in women taking HRT will increase with age.
Other states
Estrogens cause fluid retention in the body. Patients with heart or kidney failure should be under constant medical supervision.
Careful monitoring is necessary when carrying out HRT in women with a history of hypertriglyceridemia, since in this condition, during estrogen therapy, rare cases of a sharp increase in the concentration of triglycerides in the blood plasma, leading to the development of pancreatitis, have been described.
Estrogens increase the concentration of thyroxine-binding globulin, leading to an increase in the total concentration of circulating thyroid hormones. The concentrations of free T3 and T4 do not change.
The content of other proteins, such as corticosteroid binding globulin and sex hormone binding globulin, may increase, which may lead to an increase in the total concentration of circulating glucocorticoids and sex hormones, respectively. The concentrations of free or biologically active hormones do not change. It is also possible to increase the content of other blood plasma proteins (angiotensinogen (renin substrate), alpha-1-antitrypsin, ceruloplasmin).
Chloasma
In some cases, chloasma may develop, especially in women with a history of chloasma during pregnancy. Women who are prone to developing chloasma should minimize exposure to sunlight or ultraviolet radiation while using HRT.
Effect on cognitive function
HRT does not improve cognitive function. The WHI study showed a trend towards a possible increased risk of dementia in women who started long-term HRT with combined estrogen-progestin drugs or estrogen alone after the age of 65 years.
Impact on the ability to drive vehicles and machinery
The effect of estrogens on the ability to drive vehicles and operate machinery has not been studied.
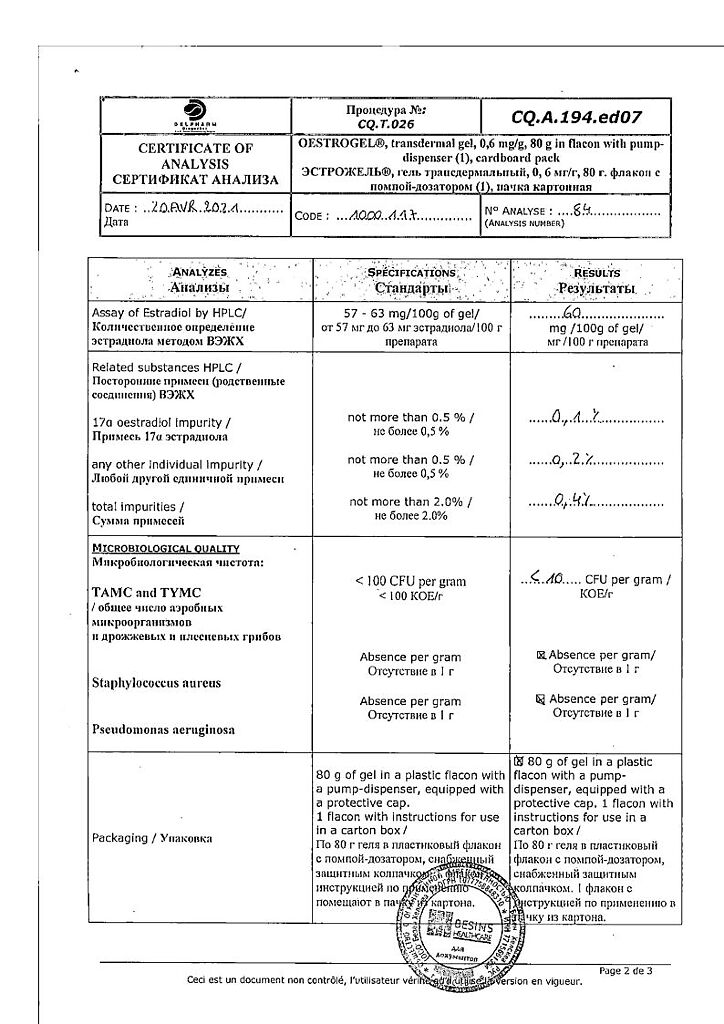
Active ingredient
Estradiol
Composition
1 g
1 dose (2.5 g gel)
estradiol (as hemihydrate)
600 mcg
1.5 mg
Excipients:
carbomer (carbopol 980) – 5 mg,
trolamine (triethanolamine) – 5 mg,
ethanol – 400 mg,
purified water – q.s. up to 1 year
Pregnancy
Estrogel is contraindicated for use during pregnancy and breastfeeding.
Contraindications
– breast cancer (diagnosed, suspected or history);
– diagnosed or suspected estrogen-dependent malignant tumors of the genital organs (for example, endometrial cancer) or a history of them;
– bleeding from the genital tract of unknown etiology;
– untreated endometrial hyperplasia;
– identified acquired or hereditary predisposition to venous or arterial thrombosis, including antithrombin III deficiency, protein C deficiency, protein S deficiency);
– venous thrombosis and thromboembolism currently or in history (including deep vein thrombosis and thrombophlebitis, pulmonary embolism);
– active or recently suffered arterial thromboembolic diseases (including angina pectoris, myocardial infarction);
– acute liver disease or a history of liver disease if the results of liver function tests have not returned to normal;
– congenital hyperbilirubinemia (Gilbert, Dubin-Johnson, Rotor syndromes);
– benign or malignant liver tumors currently or in history;
– cholestatic jaundice or severe cholestatic itching (including during previous pregnancy or while taking sex hormones);
– pregnancy;
– lactation period (breastfeeding);
– porphyria;
– hypersensitivity to estradiol and/or any of the excipients of the drug.
With caution: the drug should be used for diseases and conditions such as: uterine fibroids; endometriosis; history of endometrial hyperplasia; the presence of risk factors for estrogen-dependent tumors (breast cancer in first-degree relatives); presence of risk factors for thromboembolic disorders; arterial hypertension; liver diseases (including liver adenoma) with normal liver function tests; gallbladder diseases (including cholelithiasis); diabetes mellitus with or without diabetic angiopathy; migraine or severe headache; systemic lupus erythematosus; epilepsy; bronchial asthma; otosclerosis, chronic heart failure; renal failure; IHD; sickle cell anemia; history of chloasma; history of hypertriglyceridemia; pancreatitis; hereditary angioedema.
Experience in treating women over 65 years of age is limited.
Side Effects
Possible side effects of HRT are described below. The frequency of side effects is determined as follows: often (>1/100; 1/1000; 1/10,000; <1/1000).
From the immune system: rarely – anaphylactic reactions (in women with a history of allergic reactions).
Metabolism and nutrition: rarely – impaired glucose tolerance.
Mental disorders: infrequently – depression, mood swings; rarely – changes in libido.
From the nervous system: often – headache; infrequently – migraine, dizziness; rarely – exacerbation of epilepsy.
From the cardiovascular system: infrequently – venous thromboembolism; rarely – increased blood pressure.
From the gastrointestinal tract: often – nausea, abdominal pain; infrequently – flatulence, vomiting.
From the liver and biliary tract: rarely – deviation from the norm in liver function tests.
From the skin and subcutaneous tissues: infrequently – itching; rarely – skin discoloration, acne.
From the genital organs and mammary gland: often – swelling of the mammary glands, pain in the mammary glands, enlargement of the mammary glands, dysmenorrhea, menorrhagia, metrorrhagia, leukorrhea, endometrial hyperplasia; uncommon – benign tumors of the mammary glands, enlarged uterus, uterine fibroids, vaginitis, candidal vaginitis; rarely – galactorrhea.
Other: often – changes in body weight (decrease or increase), fluid retention with peripheral edema; infrequently – asthenia.
Other adverse reactions identified during therapy with estrogen-gestagen drugs:
gallbladder diseases;
from the skin and subcutaneous tissues: chloasma, erythema multiforme, erythema nodosum, thrombocytopenic purpura;
increased risk of developing dementia over the age of 65.
Breast cancer risk
Women using combined estrogen-progestin drugs for more than 5 years have a 2-fold increase in the risk of being diagnosed with breast cancer. When carrying out HRT with estrogen only, the risk of developing breast cancer is significantly lower than when carrying out HRT with combined estrogen-progestogen drugs. The risk of developing breast cancer depends on the duration of HRT.
Endometrial cancer risk
In postmenopausal women with an intact uterus
The incidence of endometrial cancer is about 5 cases in every 1000 women with an intact uterus who do not undergo HRT.
In women with an intact uterus, HRT with estrogen alone is not recommended, as this increases the risk of developing endometrial cancer.
Depending on the duration of estrogen-only use and dose, the increased risk of endometrial cancer in epidemiological studies ranged from 5 to 55 additional cases diagnosed in every 1000 women aged 50 to 65 years.
Adding a progestin for at least 12 days of the cycle to estrogen-only therapy may prevent this increased risk. In the WHI study, when carrying out HCG (sequential or continuous) with combined estrogen-progestin drugs for five years, there was no increase in the risk of endometrial cancer (RR 1.0 (0.8-1.2)).
Ovarian cancer
Long-term use of estrogen-only and combined estrogen-progestin HRT was associated with a small increase in the risk of developing ovarian cancer. The WHI study found 1 additional case of ovarian cancer per 2,500 women with HRT over five years.
Risk of venous thromboembolism
Women receiving HRT have an increased risk of developing venous thromboembolism (VTE), in particular deep vein thrombosis or pulmonary embolism, compared with women not receiving HRT by 1.3-3 times. The likelihood of developing VTE is higher in the first year of HRT than in subsequent years.
Interaction
The use of Estrogel® in combination with surfactants (for example, sodium lauryl sulfate) or other substances that change the structure or barrier function of the skin may reduce its effectiveness. Therefore, it is necessary to avoid combined use of the drug with strong detergents and detergents (for example, containing benzalkonium or benzethonium chloride), skin care products with a high ethanol content (astringents, sunscreens) and keratolytic agents (for example, salicylic or lactic acid).
The use of any concomitant medications that are harmful to the skin (for example, cytotoxic) should be avoided.
The metabolism of estradiol is accelerated when used simultaneously with inducers of microsomal liver enzymes, such as antiepileptic drugs (phenobarbital, phenytoin, carbamazepine); some antibiotics and antiviral drugs (rifampicin, rifabutin, nevirapine, efavirenz); herbal preparations containing St. John’s wort.
Ritonavir and nelfinavir, also known as strong inhibitors, when used together with sex hormones, on the contrary, exhibit inducing properties.
With transdermal use, it is possible to avoid the “first pass” effect through the liver, thus, the effect of drugs for HRT with transdermal application of estrogens, perhaps to a lesser extent than with oral use, depends on the action of inducers of microsomal liver enzymes.
The metabolism of estradiol is accelerated when used simultaneously with tranquilizers (anxiolytics), narcotic analgesics, and anesthetics. The concentration of estradiol in blood plasma is also reduced when used simultaneously with certain antibiotics (penicillins and tetracyclines).
The effect of estradiol is enhanced by taking folic acid and thyroid hormone preparations.
In clinical practice, increased estrogen metabolism can lead to a weakening of the effect and changes in the nature of uterine bleeding.
Estradiol increases the effectiveness of lipid-lowering drugs.
Estradiol weakens the effect of male sex hormones, hypoglycemic, diuretic, antihypertensive drugs and anticoagulants.
Overdose
No symptoms of acute overdose have been reported. Pain in the mammary glands or excess production of cervical secretions may indicate that the dose of the drug is too high.
Symptoms of estrogen overdose may include nausea and withdrawal bleeding.
Treatment: there is no specific antidote; it is necessary to discontinue the drug and carry out symptomatic therapy.
Recommendations for use
Estrogel® is prescribed externally, continuously or cyclically. Doses and duration of therapy are determined individually.
Typically, the initial dose of the drug is 2.5 g of gel 1 time / day, which corresponds to 1.5 mg of estradiol. For most patients, this dose is effective in relieving menopausal symptoms. If after one month of therapy effectiveness is not achieved, it is possible to increase the daily dose of the drug to a maximum of 5 g of gel, which corresponds to 3 mg of estradiol.
To initiate and continue treatment for menopausal symptoms, the minimum effective dose should be used for the minimum period of time.
For the prevention of osteoporosis in postmenopausal women, the minimum effective dose in most patients is 2.5 g of Estrogel® 1 time/day.
When using the drug in a tube, a plastic dispenser applicator is used to determine the daily dose: 1 dose of the applicator corresponds to 2.5 g of gel (which corresponds to 1.5 mg of estradiol).
When using the drug in a bottle, one press on the dispenser pump releases 1.25 g of gel (which corresponds to 0.75 mg of estradiol), equal to half the daily dose. The average daily dose of the drug is 2.5 g of gel (2 clicks on the dispenser pump).
The use of Estrogel® without the addition of a gestagen is possible only in patients with a removed uterus.
For patients with an intact (unremoved) uterus, it is recommended to prescribe a gestagen during treatment with Estrogel®.
During the menopausal transition, treatment should be carried out for at least 3 weeks in a row, followed by a break of 1 week, while gestagen is administered orally during the last 12-14 days of the month.
During perimenopause, treatment can be carried out from the 1st to the 25th day of the month simultaneously with oral administration of gestagen. During a week-long break, menstrual-like bleeding may occur due to a decrease in the content of sex hormones. It is recommended to use only those gestagens that can be taken simultaneously with estrogens.
During the postmenopausal period, treatment with estrogens in combination with gestagens is carried out continuously.
Long-term estrogen monotherapy is indicated in women after hysterectomy. In women who have undergone hysterectomy, the addition of a progestin in the absence of a history of endometriosis is not recommended.
Depending on the clinical symptoms, after 2-3 cycles of treatment, the dose is adjusted:
when symptoms of hyperestrogenism appear, such as a feeling of tension in the mammary glands, a feeling of fullness in the abdomen and pelvis, anxiety, nervousness, aggressiveness, a dose reduction is necessary;
with symptoms of hypoestrogenism, such as persistent hot flashes, dry vaginal mucosa, headache, sleep disturbances, asthenia, tendency to depression, the dose should be increased.
In women who have not previously used drugs for HRT, and in women switching to Estrogel® from a combination drug for HRT with a continuous regimen, treatment with Estrogel® can be started on any day convenient for the patient. In women switching to Estrogel® from a continuous sequential HRT regimen, treatment should begin after completion of the previous regimen.
If the patient has forgotten to apply the gel, this should be done as soon as possible, but no later than within 12 hours from the date of application of the drug. If more than 12 hours have passed, the application of Estrogel® should be postponed until the next time. If the drug is not used regularly (missed doses), breakthrough bleeding and spotting may occur.
Directions for use
The gel is applied by patients independently, in the morning or evening, in a thin layer on clean, dry skin of the abdomen, lumbar region, shoulders or forearms until completely absorbed. The application area should be at least the area of 2 palms.
Do not massage the area where the gel is applied. It is necessary to avoid getting the gel on the mammary glands and the mucous membrane of the vulva and vagina.
Application is considered correct and effective if the gel is completely absorbed within 2-3 minutes.
If the sticky consistency persists for more than 5 minutes after application, it means that too little skin surface is covered with the gel.
Estrogel® does not leave stains.
You should wash your hands immediately after applying the gel.
Application of the drug in a tube. Open the tube and pierce the metal membrane of the tube using the small punch located at the top of the tube lid. The required dose is removed from the tube along the applicator ruler.
1 dose corresponds to a column of extracted gel with a diameter corresponding to the diameter of the tube outlet, the length of which coincides with the recess on the applicator ruler. The recess has a line that allows you to divide the daily dose in half. One tube of gel is designed for 30 doses.
Application of the drug in the bottle. It is necessary to remove the cap from the bottle and press firmly on the dispenser pump, placing your other hand to collect the gel. The dose released the first time you press may not be accurate. It is recommended to throw it away. The bottle is designed for 64 pumps. After 64 presses, the amount of gel released per press may be less than desired. Therefore, it is not recommended to use the bottle after 64 clicks on the dispenser pump.
Functional features
Suction and distribution
When the gel is applied topically to a large surface of the skin, triethanolamine evaporates and approximately 10% of estradiol is absorbed through the skin into the vascular system, regardless of the patient’s age. Daily use of the drug Estrogel® in a dose of 2.5 g or 5 g over an area of 400-750 cm2 leads to a gradual increase in the concentration of estradiol and estrone and provides them with Css in the blood plasma after approximately 3-5 days in a ratio characteristic of the beginning of the middle follicular phase of the menstrual cycle.
When using the drug Estrogel® in 17 postmenopausal women 1 time/day by applying it to the back surface of one arm from wrist to shoulder for 14 days, the Cmax of estradiol and estrone in the blood plasma on the 12th day of use was 117 pg/ml and 128 pg/ml, respectively. The average concentration of estradiol and estrone in the blood plasma over a 24-hour period after administration of the drug Estrogel® at a dose of 2.5 g on the 12th day of administration was 76.8 pg/ml and 95.7 pg/ml, respectively.
Metabolism and excretion
Estradiol is metabolized mainly in the liver to estriol, estrone and their conjugated metabolites (glucuronides, sulfates). These metabolites undergo enterohepatic recirculation. After cessation of treatment, estradiol concentrations return to baseline levels after approximately 76 hours.
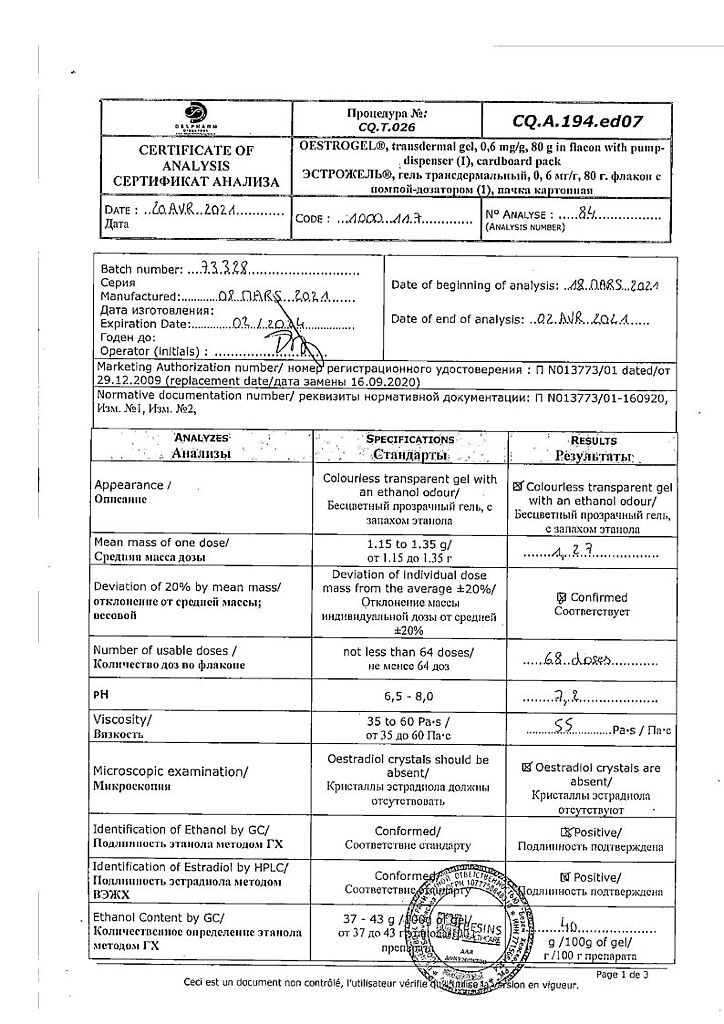
Storage conditions
List B. The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Shelf life
3 years.
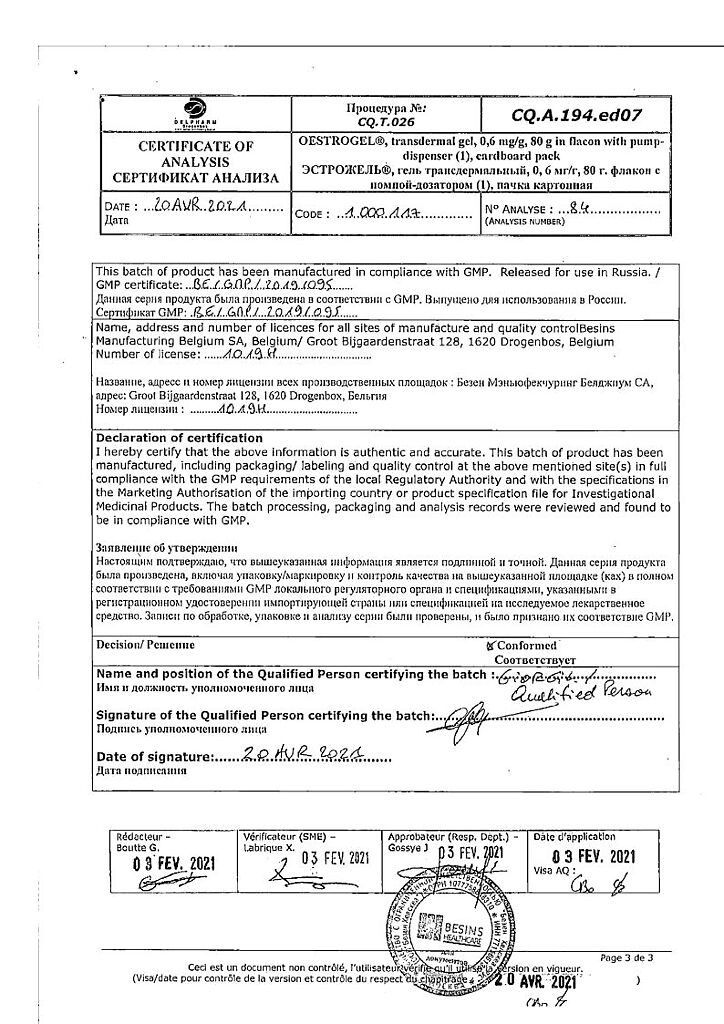
Manufacturer
Bezen Manufacturing Belgium S.A., Belgium
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | List B. The drug should be kept out of reach of children at a temperature not exceeding 25°C. |
| Manufacturer | Delpharm Drogenbos SA, Belgium |
| Medication form | transdermal gel |
| Brand | Delpharm Drogenbos SA |
Related products
Buy Oestrogel, transdermal gel 600 mcg/g 80 g with delivery to USA, UK, Europe and over 120 other countries.