No products in the cart.
Description
Skin itching, Allergic conjunctivitis, Allergies, Allergic rhinitis, UrticariaAllergic rhinitis, allergic conjunctivitis, urticaria.
Indications
Indications
Allergic rhinitis, allergic conjunctivitis, urticaria.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Special instructions
Special instructions
Ebastine may interfere with allergy skin test results. Therefore, it is recommended to carry out such tests no earlier than 5-7 days after discontinuation of the drug.
During therapy, caution should be exercised in the presence of a prolonged QT interval on the electrocardiogram, hypokalemia, as well as while taking azole antifungals and macrolide antibiotics (see section “Interaction with other drugs”).
Caution should be exercised when taking the drug in patients with severe liver failure (see section “Method of administration and dosage”).
Impact on the ability to drive vehicles. Wed and fur.:
In therapeutic doses it does not affect the ability to drive vehicles and machinery.
In case of side effects from the central nervous system, such as drowsiness, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
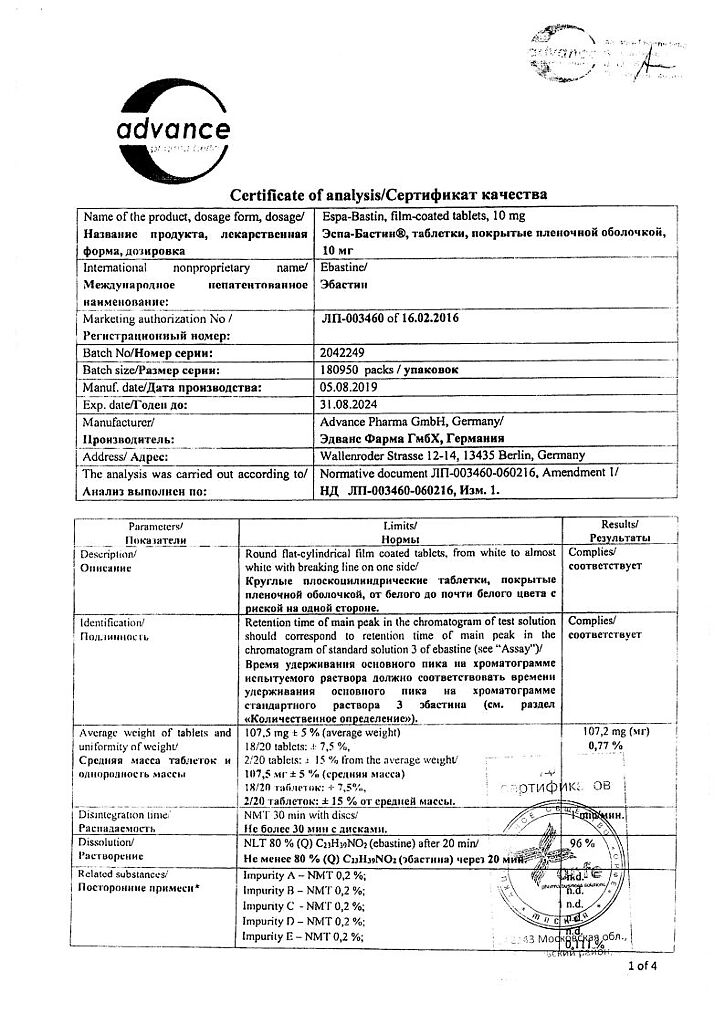
Active ingredient
Active ingredient
Ebastine
Composition
Composition
Each film-coated tablet contains:
Tablet core:
active substance:
ebastine – 10.00 mg/20.00 mg;
excipients:
microcrystalline cellulose – 85.00 mg/171.00 mg,
sodium carboxymethyl starch (type A) – 3.00 mg/6.00 mg,
colloidal silicon dioxide anhydrous – 1.00 mg/2.00 mg,
magnesium stearate – 0.50 mg/1.00 mg.
Film shell Opadry Y-1-7000 is white, consisting of: hypromellose-5cP – 4.69 mg/9.38 mg, titanium dioxide – 2.34 mg/4.68 mg, macrogol 400 – 0.47 mg/0.94 mg.
Pregnancy
Pregnancy
The safety of the drug in pregnant women has not been studied, therefore taking Espa-Bastin® during pregnancy is contraindicated.
It is not recommended to take the drug while breastfeeding.
Contraindications
Contraindications
Hypersensitivity to the active substance or other components of the drug;
severe liver dysfunction (class C according to the Child-Pugh classification);
pregnancy;
lactation period;
children’s age (up to 12 years).
With caution:
Prolongation of the QT interval on the ECG, hypokalemia, renal and/or mild to moderate liver failure (class A, B according to the Child-Pugh classification).
The drug should be used with caution when taken simultaneously with ketoconazole or itraconazole and erythromycin – there may be an increased risk of prolongation of the QT interval on the ECG.
Side Effects
Side Effects
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (≥ 1/10), common (≥ 1/100, < 1/10), uncommon (≥ 1/1000, < 1/100), rare (≥ 1/10000, < 1/1000) and very rare (< 1/10000); frequency unknown (the frequency of events cannot be determined from the available data).
Gastrointestinal disorders: often: dryness of the oral mucosa; rarely: nausea, abdominal pain, dyspepsia; very rarely: vomiting.
Nervous system disorders: often: drowsiness, headache; very rarely: nervousness, insomnia, dizziness, paresthesia, dysesthesia.
Disorders of the liver and biliary tract: very rarely: hepatitis, cholestasis, changes in laboratory parameters (increased activity of liver transaminases, GGT, alkaline phosphatase, bilirubin).
Immune system disorders: frequency unknown: hypersensitivity reactions (eg, anaphylaxis, angioedema).
Cardiovascular system disorders: very rare: tachycardia, palpitation.
Skin and subcutaneous tissue disorders: very rare: urticaria, rash, dermatitis.
Reproductive system disorders: very rare: menstrual disorders.
General and local reactions: very rarely: edema, asthenic syndrome.
Interaction
Interaction
It is not recommended to prescribe concomitantly with ketoconazole or itraconazole and erythromycin (increased risk of QT prolongation).
There were no clinically significant interactions with theophylline, indirect anticoagulants, cimetidine, diazepam, ethanol and ethanol-containing drugs.
Rifampicin reduces the concentration of ebastine in the blood plasma and has an inhibitory effect on the antihistamine effect of ebastine.
May enhance the effect of other antihistamines.
Overdose
Overdose
When taking up to 100 mg of ebastine per day, no clinically significant symptoms of overdose are observed.
A specific antidote has not been identified.
Treatment: gastric lavage, monitoring vital functions of the body, including ECG monitoring, symptomatic therapy.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Advance Pharma GmbH, Germany
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Store out of the reach of children. |
| Manufacturer | Edwans Pharma GmbH, Germany |
| Medication form | pills |
| Brand | Edwans Pharma GmbH |
Other forms…
Related products
Buy Espa-Bastin, 10 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.