No products in the cart.
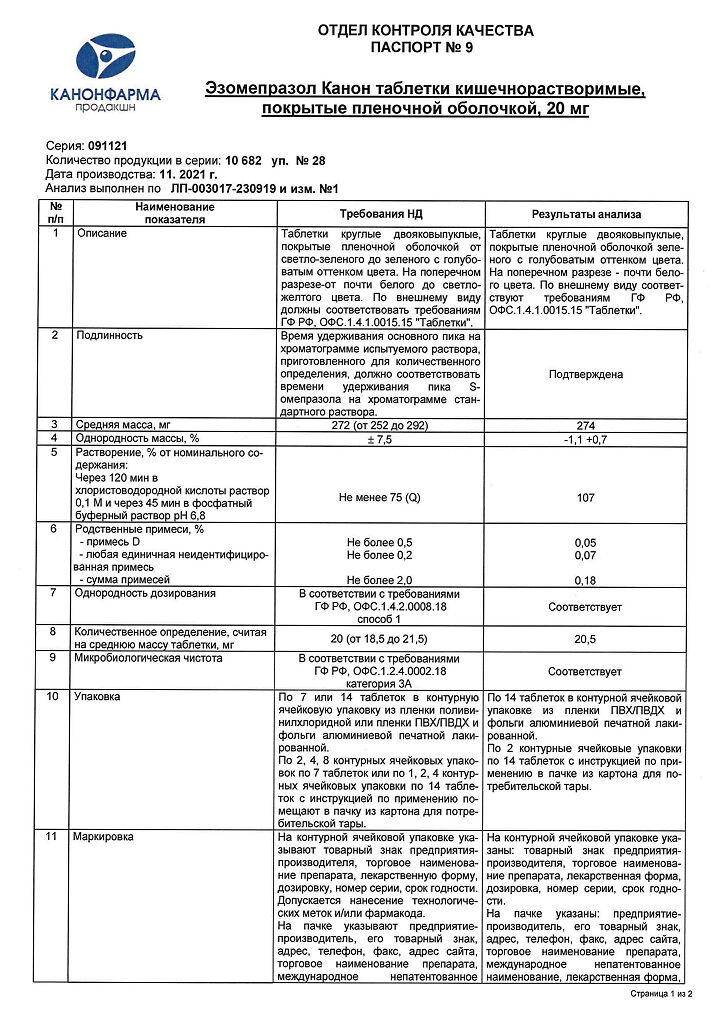
Esomeprazole Canon, 20 mg 28 pcs
€7.00 €6.33
Out of stock
(E-mail when Stock is available)
EAN: 4606486023364
SKU: 275686
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Esomeprazole is the S-isomer of omeprazole and reduces gastric hydrochloric acid secretion by specifically inhibiting the proton pump in the parietal cells of the stomach. S- and R-isomers of omeprazole have similar pharmacodynamic activity.
Mechanism of action
Esomeprazole is a weak base converted to active form in strongly acidic environment of secretory tubules of parietal cells of gastric mucosa and inhibits proton pump – H+/K+ – ATPase enzyme, thus inhibiting both basal and stimulated hydrochloric acid secretion.
Effect on hydrochloric acid secretion in the stomach
After oral administration of 20 mg or 40 mg, the effect of esomeprazole develops within 1 hour. At a daily dose of 20 mg once daily for 5 days the average maximum concentration of hydrochloric acid after pentagastrin stimulation is decreased by 90% (when measuring acid concentration 6-7 hours after taking the drug on the 5th day of therapy).
In patients with gastroesophageal reflux disease (GERD) and the presence of clinical symptoms after 5 days of daily oral administration of esomeprazole at a dose of 20 mg or 40 mg, intragastric pH values above 4.0 were maintained for an average of 13 and 17 hours out of 24 hours. Against the background of esomeprazole administration in the dose of 20 mg per day, the intragastric pH value above 4.0 was maintained for at least 8, 12 and 16 hours in 76%, 54% and 24% of patients, respectively.
The correlation between the drug concentration in plasma and inhibition of hydrochloric acid secretion was revealed (to estimate the concentration we used AUC parameter – area under the curve “concentration – time”.
Therapeutic effect, reached as a result of inhibiting hydrochloric acid secretion
At the use of the drug in a dose of 40 mg healing of reflux esophagitis occurs approximately in 78% of patients after 4 weeks of therapy and in 93% of patients after 8 weeks of therapy.
Treatment with esomeprazole at a dose of 20 mg twice daily in combination with appropriate antibiotics for one week results in successful eradication of Helicobacter pylori in approximately 90% of patients.
Patients with uncomplicated peptic ulcer disease after one week of eradication do not require subsequent monotherapy with drugs that reduce gastric gland secretion for ulcer treatment and elimination of symptoms.Esomeprazole has been shown to be effective in peptic ulcer bleeding confirmed endoscopically.
Other effects related to inhibition of hydrochloric acid secretion
During treatment with drugs that reduce gastric gland secretion, plasma gastrin concentration increases as a result of reduced hydrochloric acid secretion. The decrease in hydrochloric acid secretion increases the concentration of chromogranin A (CgA). Increased concentration of CgA may affect the results of examinations for detection of neuroendocrine tumors. To prevent this effect, esomeprazole should be temporarily discontinued 5 days before CgA testing. In patients receiving esomeprazole for a long time, an increase in enterochromaffin-like cells has been observed, probably related to an increase in plasma gastrin concentration.
The formation of glandular cysts in the stomach is more frequently observed in patients taking drugs that reduce gastric gland secretion over a long period of time. These phenomena are due to physiological changes resulting from marked inhibition of hydrochloric acid secretion. The cysts are benign and undergo reverse development.
The use of drugs that inhibit the secretion of hydrochloric acid in the stomach, including proton pump inhibitors, is accompanied by an increase in the gastric content of microbial flora normally present in the gastrointestinal tract. Use of proton pump inhibitors may lead to a slight increase in the risk of gastrointestinal tract infections caused by bacteria of the genus Salmonella spp. and Campylibacter spp. and probably Clostridium difficile in hospitalized patients.
In two comparative studies with ranitidine, esomeprazole showed better efficacy with respect to the treatment of gastric ulcers in patients receiving nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors.
Indications
Indications
Gastroesophageal reflux disease (GERD)
Long-term supportive treatment after healing of erosive reflux-esophagitis, prevention of relapses;
Symptomatic treatment of GERD.
Gastric and duodenal ulcer
in combination therapy:
Treatment of duodenal ulcer associated with Helicobacter pylori;
Prevention of recurrence of peptic ulcer associated with Helicobacter pylori.
Long-term acid-suppressive therapy in patients with peptic ulcer bleeding (after intravenous application of drugs that decrease gastric gland secretion, to prevent recurrence).
Patients taking NSAIDs for a long time
Treatment of gastric ulcers caused by NSAIDs consumption;
Prevention of gastric and duodenal ulcers caused by NSAIDs consumption in patients belonging to the risk group.
Zollinger-Ellison syndrome or other conditions characterized by pathological hypersecretion of gastric glands, including idiopathic hypersecretion.
Active ingredient
Active ingredient
Composition
Composition
Dosage 20 mg
1 film-coated, enteric-soluble tablet contains:
active ingredient:
esomeprazole magnesium dihydrate 21.8 mg, in terms of esomeprazole 20 mg;
excipients:
Hyprolose low-substituted (hydroxypropyl cellulose) 14 mg,
Corn starch pregelatinized 37.2 mg,
Colloidal silicon dioxide 2 mg,
mannitol 23 mg,
sodium stearyl fumarate 2 mg,
p> microcrystalline cellulose 140 mg;
composition of the film coating:
Opadray clear 8 mg, including:
[hypromellose (hydroxypropyl methylcellulose) 6.4 mg, macrogol (polyethylene glycol) 1.6 mg]. Acrylic-Green 22 mg, including: [methacrylic acid and ethyl acrylate copolymer [1:1] 14.52 mg, colloidal silica 0.22 mg, sodium hydrocarbonate 0.22 mg, sodium lauryl sulfate 0.11 mg, iron oxide yellow 0.154 mg, indigo carmine dye 0.176 mg, brilliant blue dye 0.066 mg, talc 3.63 mg, titanium dioxide 2.904 mg]. Triethylcitrate 2 mg.
How to take, the dosage
How to take, the dosage
Ingestion. The tablet should be swallowed whole, without chewing, with plenty of water.
Adults and children from 12 years
Gastroesophageal reflux disease
Treatment of erosive reflux esophagitis: 40 mg once daily for 4 weeks.
An additional 4-week course of treatment is recommended if esophagitis does not heal after the first course or if symptoms persist.
Long-term maintenance treatment after healing of erosive reflux esophagitis, prevention of relapses: 20 mg once a day. Symptomatic treatment of GERD: 20 mg once daily in patients without esophagitis. If symptoms do not disappear after 4 weeks of treatment, further evaluation of the patient should be carried out. After elimination of symptoms, it is possible to switch to the regimen of taking the drug “as needed” – 20 mg once a day if symptoms recur. For patients taking NSAIDs and those at risk of gastric or duodenal ulcer, treatment on an “as needed” regimen is not recommended.
Adults
Gastric and duodenal ulcer
As part of combination therapy for eradication of Helicobacter pylori:
Long-term acid suppressive therapy in patients who have had bleeding from a peptic ulcer (after intravenous use of drugs that reduce gastric gland secretion, to prevent recurrence): Esomeprazole Canon 40 mg once daily for 4 weeks after intravenous use of gastric gland-lowering drugs.
Patients taking long-term NSAIDs
Treatment of gastric ulcers due to NSAIDs: Esomeprazole Canon 20 mg once daily; treatment duration 4-8 weeks.
Prevention of gastric and duodenal ulcers caused by taking NSAIDs: Esomeprazole Canon 20 mg once daily.
Conditions characterized by pathological hypersecretion of gastric glands, including Zollinger-Ellison syndrome and idiopathic hypersecretion: Recommended initial dose of Esomeprazole Canon 40 mg 2 times daily. Further, the dose is adjusted individually; the duration of treatment is determined by the clinical picture of the disease.
There is experience of using from 80 to 160 mg of esomeprazole per day; when taking the drug more than 80 mg per day, it is recommended to divide the required dose into 2 doses.
Renal failure: Dose adjustment of Esomeprazole Canon is not required. However, the experience of using esomeprazole in patients with severe renal failure is limited; therefore, caution should be exercised when prescribing the drug in this category of patients.
Hepatic failure: No dose adjustment is required in mild to moderate hepatic failure. For patients with severe hepatic insufficiency, the maximum daily dose should not exceed 20 mg.
Elderly patients: No dose adjustment is required.
Interaction
Interaction
The effect of esomeprazole on the pharmacokinetics of other drugs
Reduced gastric hydrochloric acid secretion during treatment with esomeprazole and other proton pump inhibitors may lead to changes in absorption of drugs whose absorption depends on the acidity of the environment. Like antacids and other drugs that reduce the acidity of gastric juice, the use of esomeprazole may lead to decreased absorption of ketoconazole, itraconazole and erlotinib, and increased absorption of drugs such as digoxin.
Simultaneous administration of esomeprazole in a dose of 20 mg once daily and digoxin increases digoxin bioavailability by 10% (digoxin bioavailability was increased by up to 30% in two out of 10 patients).
It is known about interaction of esomeprazole with some antiretroviral drugs. The mechanisms and clinical significance of these interactions are not always known. Increased pH value on esomeprazole therapy may affect absorption of antiretroviral drugs. Interaction at the level of CYP2C19 isoenzyme is also possible.
If esomeprazole and some antiretroviral drugs such as atazanavir and nelfinavir are coadministered during esomeprazole therapy, a decrease in their serum concentrations is noted.
Concomitant administration of esomeprazole 40 mg once daily and atazanavir 300 mg/ritonavir 100 mg in healthy volunteers resulted in a significant reduction of atazanavir bioavailability (AUC and maximal and minimal concentrations decreased by approximately 75%). Increasing the atazanavir dose to 400 mg did not compensate for the effect of esomeprazole on atazanavir bioavailability.
Simultaneous use of esomeprazole and saquinavir resulted in increased serum saquinavir concentrations; when used with some other antiretrovirals their concentrations were unchanged. Given the similar pharmacokinetic and pharmacodynamic properties of omeprazole and esomeprazole, co-administration of esomeprazole with antiretrovirals such as atazanavir and nelfinavir is not recommended.
Esomeprazole inhibits the CYP2C19 isoenzyme, the main enzyme involved in its metabolism. Accordingly, co-administration of esomeprazole with other drugs whose metabolism involves the CYP2C19 isoenzyme, such as diazepam, citalopram, imipramine, clomipramine, phenytoin and others, may lead to increased plasma concentrations of these drugs, which in turn may require dose reduction. This interaction is particularly important to keep in mind when prescribing Esomeprazole Canon on an “as needed” regimen. When co-administration of 30 mg of esomeprazole and diazepam, which is a substrate of CYP2C19 isoenzyme, a 45% decrease in diazepam clearance was noted.
Administration of esomeprazole in a dose of 40 mg resulted in a 13% increase in residual concentration of phenytoin in patients with epilepsy. In this regard, it is recommended to monitor plasma phenytoin concentrations at the beginning of treatment with esomeprazole and upon its withdrawal.
Concomitant use of esomeprazole at a dose of 40 mg leads to an increase in plasma phenytoin concentrations by 13% in patients with epilepsy.
It is recommended to monitor plasma concentrations of phenytonin at the beginning of therapy with esomeprazole and at its withdrawal.
Administration of esomeprazole in dose 40 mg once daily increases AUC and TCmax of voriconazole (substrate of CYP2C19 isoenzyme) by 15% and 41% respectively.
Concomitant administration of warfarin and esomeprazole 40 mg does not lead to changes in coagulation time in patients taking long-term warfarin. However, several cases of clinically significant increases in MHO (international normalized ratio) have been reported when warfarin and esomeprazole are coadministered. It is recommended to monitor MHO at the beginning and at the end of coadministration of esomeprazole and warfarin or other coumarin derivatives.
Coadministration of cisapride with 40 mg of esomeprazole leads to increased values of pharmacokinetic parameters of cisapride in healthy volunteers: AUC – by 18% and T1/2 by 26%, for one of the active metabolites of cisapride the increase was 29% and 69% respectively.
Simultaneous use of esomeprazole in dose of 40 mg with cisapride increased pharmacokinetic parameters of cisapride in healthy volunteers: AUC – by 32% and T1/2 – by 31%, but Cmax was not significantly changed.
Slight prolongation of QT interval, which was observed with cisapride monotherapy, was not increased with the addition of esomeprazole. some patients showed increased serum concentration of methotrexate during concomitant use with proton pump inhibitors. When using high doses of methotrexate, temporary withdrawal of esomeprazole should be considered.
Esomeprazole does not cause clinically significant changes in pharmacokinetics of amoxicillin and quinidine.Studies evaluating short-term co-administration of esomeprazole and naproxen or rofecoxib did not reveal a clinically
significant pharmacokinetic interaction.
Simultaneous short-term use of esomeprazole and naproxen or rofecoxib showed no clinically significant pharmacokinetic interaction.
In a clinical study, interactions were studied when clopidogrel (300 mg loading dose, then 75 mg/day) was used with esomeprazole (80 mg) at the same time for 5 days. The thiol metabolite (active metabolite) activity of clopidogrel was reduced by 46% (day 1 of therapy) and 42% (day 5 of therapy), when clopidogrel and omeprazole were taken at the same time. When clopidogrel and esomeprazole were taken at the same time, the average suppression of platelet aggregation (IRA) was reduced by 47% (during 24 hours of therapy) and 30% (day 5 of therapy).According to another study: esomeprazole when used with clopidogrel not at the same time, at different times, does not have inhibitory effect on CYP2C19 isoenzyme. Studies have reported inconsistent data of clinical manifestations of interaction with clopidogrel on the cardiovascular system.
Simultaneous use with tacrolimus may increase serum concentrations of tacrolimus.
Effect of drugs on pharmacokinetics of esomeprazole
CYP2C19 and CYP3A4 isoenzymes are involved in metabolism of esomeprazole.
Concomitant use of esomeprazole with clarithromycin (500 mg 2 times per day) which inhibits CYP3A4 isoenzyme results in 2-fold increase of esomeprazole AUC.
The combined use of esomeprazole and a combined CYP3A4 and CYP2C19 isoenzyme inhibitor such as voriconazole may lead to more than a 2-fold increase in the AUC of esomeprazole. In such cases there is usually no need to adjust the dose of esomeprazole.
Medicinal products inducing CYP2C19 and CYP3A4 isoenzymes, such as rifampicin and preparations of Saint John’s wort, when used simultaneously with esomeprazole may lead to a decrease in plasma concentration of esomeprazole due to accelerated metabolism of esomeprazole.
Special Instructions
Special Instructions
In the presence of any alarming symptoms (such as significant spontaneous weight loss, recurrent vomiting, dysphagia, bloody vomiting, or melena) and the presence of a peptic ulcer (or suspected peptic ulcer), malignancy should be excluded, since treatment with esomeprazole may result in flattening of symptoms and delay diagnosis.
Patients taking the drug for a long period (especially over a year) should be under regular medical supervision.
Patients taking esomeprazole “as needed” should be instructed to contact their physician if their symptoms change. Taking into account the fluctuations in plasma concentrations of esomeprazole when prescribing therapy “as needed”, the interaction of the drug with other medicinal products should be considered (see section “Interaction with other medicinal products and other types of drug interaction”).
When using esomeprazole for eradication of Helicobacter pylori the possibility of drug interactions for all components of triple therapy should be considered. Clarithromycin is a potent inhibitor of CYP3A4 isoenzyme, therefore when using eradication therapy in patients receiving other drugs metabolized with participation of CYP3A4 isoenzyme (for example, cisapride), possible contraindications and interactions of clarithromycin with these drugs should be considered.
When using proton pump inhibitors, especially when used in high doses and for a prolonged period (more than 1 year) there is a possible risk of fracture of femoral neck, carpal and vertebral bones (especially in elderly patients). Formation of glandular cysts in the stomach, decreased absorption of vitamin B12, development of hypomagnesemia are also noted.
Influence on ability to drive vehicles and mechanisms
During the treatment dizziness, blurred vision and somnolence may occur, therefore caution should be exercised while driving motor transport and engaging in other potentially dangerous activities that require high concentration and quick psychomotor reactions.
Contraindications
Contraindications
– Hypersensitivity to esomeprazole, substituted
benzimidazoles or other components of the drug;
– Children under 12 years of age (due to the lack of data on
efficacy and safety of the drug in this group of patients);
– Children aged 12 to 18 years old for all indications, except for GERD;
– Concomitant use with atazanavir and nelfinavir.
With caution
Severe renal insufficiency (experience of use is limited).
Side effects
Side effects
The World Health Organization (WHO) classification of the frequency of side effects:
very often – >1/10 appointments (>10%)
often – >1/100 to < 1/10 appointments (>1% and <10%)
infrequent – >1/1000 to < 1/100 appointments (>0.1% and < 1%)
rarely – from >1/10000 to < 1/1000 appointments (>0.01% and <0.1%)
very rare – <1/10000 appointments (<0.01%)
frequency unknown – cannot be estimated based on available data.
In each group, adverse effects are presented in decreasing order of severity.
Nervous system disorders
Frequently: headache.
Infrequent: drowsiness, insomnia, dizziness, paresthesias.
Rare: agitation, confusion, depression.
Very rare: aggressive behavior, hallucinations.
Respiratory system disorders
Rare: bronchospasm.
Digestive system disorders
Often: abdominal pain, diarrhea, flatulence, nausea, vomiting, constipation.
Infrequent: dry mouth, increased activity of “liver” enzymes.
Rare: stomatitis, gastrointestinal candidiasis (GIT), hepatitis (with or without jaundice).
Very rare: liver failure, hepatic encephalopathy in patients with a history of liver disease, microscopic colitis.
Renal and urinary tract disorders
Very rare: interstitial nephritis.
Frequency unknown: renal failure.
Reproductive system disorders
Very rare: gynecomastia.
Musculoskeletal system disorders
Rare: arthralgia, myalgia.
Very rare: muscle weakness.
Frequency unknown: fractures of the femoral neck, carpal bones, vertebrae.
Skin disorders
Infrequent: itching, rash, urticaria, dermatitis, peripheral edema.
Rare: alopecia, photosensitization, malaise, increased sweating.
Very rarely: Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme.
Blood disorders
Rare: leukopenia, thrombocytopenia.
Very rarely: agranulocytosis, pancytopenia.
Sensory organ disorders
Infrequently: blurred vision.
Rarely: taste disorder.
Allergic reactions
Rare: hypersensitivity reactions (e.g., fever, angioedema, anaphylactic reaction/anaphylactic shock).
Laboratory and instrumental data
Rare: hyponatremia.
Very rare: hypomagnesemia, hypocalcemia due to severe hypomagnesemia, hypokalemia due to severe hypomagnesemia.
Overdose
Overdose
Esomeprazole overdose has rarely been described. Oral administration of esomeprazole at a dose of 280 mg was accompanied by general weakness and gastrointestinal symptoms. A single dose of 80 mg of esomeprazole did not cause any adverse effects.
Treatment: A specific antidote is unknown. Hemodialysis is ineffective. In case of overdose, symptomatic and general supportive therapy is recommended.
Similarities
Similarities
Additional information
| Weight | 0.030 kg |
|---|---|
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | enteric soluble tablets |
| Brand | Kanonfarma Production ZAO |
Related products
Buy Esomeprazole Canon, 20 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.