No products in the cart.
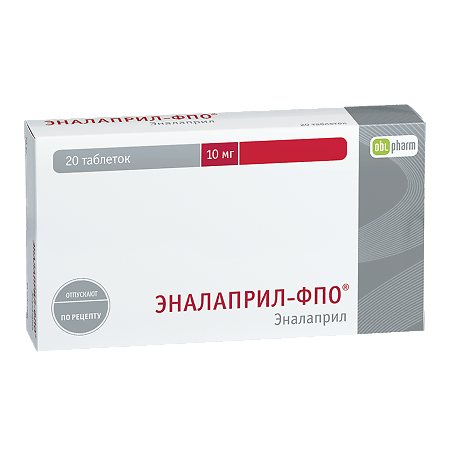
Enalapril-FPO, tablets 10 mg 20 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group:
An angiotensin-converting enzyme (ACE) inhibitor
Pharmacodynamics:
Enalapril is an antihypertensive drug, from the group of ACE inhibitors. Enalapril is a “prodrug”: its hydrolysis produces enalaprilate, which inhibits ACE. Its mechanism of action is related to the reduction of angiotensin I and angiotensin II, the reduction of which leads to a direct reduction of aldosterone secretion. This decreases total peripheral vascular resistance, systolic and diastolic blood pressure (BP), post- and preload on myocardium.
Dilates arteries to a greater extent than veins, and there is no reflex increase in heart rate.
The hypotensive effect is more pronounced at high blood plasma renin levels than at normal or reduced levels. Decrease of BP within therapeutic limits does not affect the cerebral blood flow, the blood flow in brain vessels is maintained at a sufficient level even against the background of decreased blood pressure. It enhances coronary and renal blood flow.
Long-term use reduces hypertrophy of the left ventricular myocardium and myocytes of the resistive arterial walls, prevents the progression of heart failure and slows the development of left ventricular dilatation. Improves blood supply to ischemic myocardium. Reduces platelet aggregation.
It has some diuretic effect.
The time of onset of hypotensive effect when taken orally is 1 hour, reaches a maximum after 4-6 hours and lasts up to 24 hours. In some patients, therapy for several weeks is necessary to achieve optimal blood pressure levels. In cardiac insufficiency noticeable clinical effect is observed with long-term treatment – 6 months or more.
Pharmacokinetics:
After oral administration 60% of the drug is absorbed. Food intake does not affect the absorption of enalapril.
Enalapril is up to 50% bound to plasma proteins. Enalapril is rapidly metabolized in the liver to form the active metabolite enalaprilate, which is a more active ACE inhibitor than enalapril. The bioavailability of the drug is 40%.
The maximum concentration of enalapril in blood plasma is reached after 1 hour, of enalaprilat – 3-4 hours. Enalaprilat easily passes through the histohematic barriers, excluding the blood-brain barrier, a small amount passes through the placenta and into the breast milk.
The elimination half-life of enalaprilat is about 11 hours. Enalapril is mainly excreted by the kidneys – 60% (20% as enalapril and 40% as enalaprilat), through the intestine – 33% (6% as enalapril and 27% as enalaprilat).
It is eliminated by hemodialysis (rate 62 ml/min) and peritoneal dialysis.
Indications
Indications
– arterial hypertension;
– chronic heart failure (as part of combination therapy).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Angiotensin-converting enzyme (ACE) inhibitor
Pharmacodynamics:
Enalapril is an antihypertensive drug from the group of ACE inhibitors. Enalapril is a “prodrug”: as a result of its hydrolysis, enalaprilat is formed, which inhibits ACE. The mechanism of its action is associated with a decrease in the formation of angiotensin II from angiotensin I, a decrease in the content of which leads to a direct decrease in the release of aldosterone. At the same time, total peripheral vascular resistance, systolic and diastolic blood pressure (BP), post- and preload on the myocardium decrease.
It dilates arteries to a greater extent than veins, while there is no reflex increase in heart rate.
The hypotensive effect is more pronounced with a high level of plasma renin than with a normal or reduced level. A decrease in blood pressure within therapeutic limits does not affect cerebral circulation; blood flow in the vessels of the brain is maintained at a sufficient level even against the background of reduced blood pressure. Strengthens coronary and renal blood flow.
With long-term use, hypertrophy of the left ventricle of the myocardium and myocytes of the walls of resistive arteries decreases, prevents the progression of heart failure and slows down the development of left ventricular dilatation. Improves blood supply to ischemic myocardium. Reduces platelet aggregation.
Has some diuretic effect.
The onset of the hypotensive effect when taken orally is 1 hour, reaches a maximum after 4-6 hours and lasts up to 24 hours. In some patients, therapy is necessary for several weeks to achieve an optimal level of blood pressure. In heart failure, a noticeable clinical effect is observed with long-term treatment – 6 months or more.
Pharmacokinetics:
After oral administration, 60% of the drug is absorbed. Eating does not affect the absorption of enalapril.
Enalapril is bound to plasma proteins up to 50%. Enalapril is rapidly metabolized in the liver to form the active metabolite enalaprilat, which is a more active ACE inhibitor than enalapril. Bioavailability of the drug is 40%.
The maximum concentration of enalapril in the blood plasma is achieved after 1 hour, enalaprilat – 3-4 hours. Enalaprilat easily passes through histohematic barriers, excluding the blood-brain barrier; a small amount penetrates the placenta and into breast milk.
The half-life of enalaprilat is about 11 hours. Enalapril is excreted mainly by the kidneys – 60% (20% in the form of enalapril and 40% in the form of enalaprilat), through the intestines – 33% (6% in the form of enalapril and 27% in the form of enalaprilat).
It is removed by hemodialysis (rate 62 ml/min) and peritoneal dialysis.
Special instructions
Special instructions
Caution must be exercised when prescribing to patients with reduced circulating blood volume (as a result of diuretic therapy, limiting salt intake, hemodialysis, diarrhea and vomiting) as there is an increased risk of a sudden and pronounced decrease in blood pressure after using even the initial dose of an ACE inhibitor.
Transient arterial hypotension is not a contraindication for continuing treatment with the drug after stabilization of blood pressure (BP). In case of a repeated pronounced decrease in blood pressure, the dose should be reduced or the drug discontinued.
The use of high-strength dialysis membranes increases the risk of developing an anaphylactic reaction. Correction of the dosage regimen on days free from dialysis should be carried out depending on the level of blood pressure.
Before and during treatment with ACE inhibitors, periodic monitoring of blood pressure, blood parameters (hemoglobin, potassium, creatinine, urea, liver enzyme activity), and protein in the urine is necessary.
Patients with severe chronic heart failure, coronary heart disease and cerebrovascular disease, in whom a sharp decrease in blood pressure can lead to myocardial infarction, stroke or impaired renal function, should be carefully monitored.
Sudden withdrawal of treatment does not lead to the development of rebound syndrome (a sharp rise in blood pressure).
For newborns and infants who have been exposed in utero to ACE inhibitors, it is recommended to conduct careful monitoring for the timely detection of a pronounced decrease in blood pressure, oliguria, hyperkalemia and neurological disorders, possible due to a decrease in renal and cerebral blood flow with a decrease in blood pressure caused by ACE inhibitors.
In oliguria, it is necessary to maintain blood pressure and renal perfusion by administering appropriate fluids and vasoconstrictors.
Before examining the functions of the parathyroid glands, it should be discontinued.
Alcohol enhances the hypotensive effect of the drug.
At the beginning of treatment, until the completion of the dose selection period, it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions, because Dizziness is possible, especially after the initial dose of an ACE inhibitor in patients taking diuretics.
Before surgery (including dentistry), the surgeon/anesthesiologist must be warned about the use of ACE inhibitors.
Active ingredient
Active ingredient
Enalapril
Composition
Composition
1 tablet contains the active substance – enalapril maleate 5 mg, 10 mg.
Excipients:
corn starch,
lactose (milk sugar),
microcrystalline cellulose,
colloidal silicon dioxide (Aerosil A-300),
calcium stearate.
Contraindications
Contraindications
Hypersensitivity to enalapril and other ACE inhibitors, a history of angioedema associated with treatment with ACE inhibitors, as well as hereditary or idiopathic angioedema, pregnancy, lactation, age under 18 years (efficacy and safety have not been established).
Use with caution in case of primary hyperaldosteronism, bilateral renal artery stenosis, stenosis of the artery of a single kidney, hyperkalemia, condition after kidney transplantation; aortic stenosis, mitral stenosis (with hemodynamic disturbances), idiopathic hypertrophic subaortic stenosis, systemic connective tissue diseases, coronary heart disease, cerebrovascular diseases, diabetes mellitus, renal failure (proteinuria more than 1 g / day), liver failure, in patients on a salt-restricted diet or on hemodialysis, when taken simultaneously with immunosuppressants and saluretics, in elderly people (over 65 years), inhibition of bone marrow hematopoiesis; conditions accompanied by a decrease in circulating blood volume (including diarrhea, vomiting).
Side Effects
Side Effects
Enalapril-FPO® is generally well tolerated and in most cases does not cause side effects requiring discontinuation of the drug.
From the cardiovascular system: excessive decrease in blood pressure, orthostatic collapse, rarely – chest pain, angina pectoris, myocardial infarction or stroke (usually associated with a marked decrease in blood pressure), extremely rarely arrhythmias (atrial brady- or tachycardia, atrial fibrillation), palpitations, thromboembolism of the branches of the pulmonary artery, Raynaud’s syndrome.
From the central nervous system: dizziness, headache, weakness, insomnia, anxiety, confusion, fatigue, drowsiness (2-3%), very rarely when using high doses – nervousness, depression, paresthesia.
From the senses: disorders of the vestibular apparatus, hearing and vision impairment, tinnitus.
From the digestive system: dry mouth, anorexia, dyspeptic disorders (nausea, diarrhea or constipation, vomiting, abdominal pain), intestinal obstruction, pancreatitis, impaired liver function and bile secretion, hepatitis (hepatocellular or cholestatic), jaundice.
From the respiratory system: unproductive dry cough, sore throat, hoarseness, pulmonary infiltrates, interstitial pneumonitis, bronchospasm, shortness of breath, rhinorrhea, pharyngitis.
Allergic reactions: skin rash, itching, urticaria, angioedema, extremely rarely dysphonia, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, pemphigus, photosensitivity, serositis, vasculitis, myositis, arthralgia, arthritis, stomatitis, glossitis, intestinal swelling (very rare).
From laboratory parameters: hypercreatininemia, increased urea content, increased activity of liver enzymes, hyperbilirubinemia, hyperkalemia, hyponatremia, hypoglycemia in patients with diabetes mellitus receiving oral hypoglycemic agents or insulin. In some cases, a decrease in the concentration of hemoglobin and hematocrit, an increase in ESR, thrombocytopenia, neutropenia, agranulocytosis (in patients with autoimmune diseases), and eosinophilia are noted.
From the urinary system: impaired renal function, rarely proteinuria.
Other: alopecia, decreased libido, impotence, hot flashes.
Interaction
Interaction
When enalapril is co-administered with non-steroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 inhibitors (COX-2 inhibitors), the hypotensive effect of enalapril may be reduced; with potassium-sparing diuretics (spironolactone, triamterene, amiloride) can lead to hyperkalemia; with lithium salts – to slow down the excretion of lithium (monitoring the concentration of lithium in the blood plasma is indicated).
In some patients with impaired renal function and taking NSAIDs, including COX-2 inhibitors, concomitant use of ACE inhibitors may lead to a further deterioration of renal function. These changes are reversible.
Simultaneous use with antipyretic and analgesic drugs may reduce the effectiveness of the drug.
Enalapril weakens the effect of drugs containing theophylline.
The hypotensive effect of enalapril is enhanced by diuretics, beta-blockers, methyldopa, nitrates, blockers of “slow” calcium channels of the dihydropyridine series, hydralazine, prazosin.
Immunosuppressants, allopurinol, cytostatics increase hematotoxicity. Drugs that cause bone marrow suppression increase the risk of developing neutropenia and/or agranulocytosis.
The combined use of ACE inhibitors and hypoglycemic agents (insulin, oral hypoglycemic agents) may enhance the hypoglycemic effect of the latter with the risk of developing hypoglycemia. This is most common during the first weeks of co-use and in patients with renal impairment. In patients with diabetes mellitus receiving oral hypoglycemic agents and insulin, blood glucose levels should be monitored, especially during the first month of co-administration with ACE inhibitors.
ACE inhibitors reduce the excretion of lithium by the kidneys and increase the risk of developing lithium intoxication. If it is necessary to prescribe lithium salts, monitoring the level of lithium in the blood serum is necessary.
A complex of symptoms, including facial flushing, nausea, vomiting and hypotension, has been described in rare cases with the combined use of gold preparations for parenteral use (sodium aurothiomalate) and ACE inhibitors (enalapril).
Overdose
Overdose
Symptoms: pronounced decrease in blood pressure up to the development of collapse, myocardial infarction, acute cerebrovascular accident or thromboembolic complications, convulsions, stupor.
Treatment: the patient is transferred to a horizontal position with a low headboard.
In mild cases, gastric lavage and ingestion of saline solution are indicated; in more severe cases, measures aimed at stabilizing blood pressure are indicated: intravenous administration of saline, plasma substitutes, if necessary, administration of angiotensin II, hemodialysis (enalaprilat elimination rate is 62 ml/min).
Storage conditions
Storage conditions
At a temperature not exceeding 25 ° C, out of the reach of children!
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Alium JSC, Russia
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Keep out of reach of children at a temperature not exceeding 25 °C! |
| Manufacturer | Alium JSC, Russia |
| Medication form | pills |
| Brand | Alium JSC |
Other forms…
Related products
Buy Enalapril-FPO, tablets 10 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.