No products in the cart.
Description
Pimecrolimus is a derivative of the macrolactam ascomycin and has anti-inflammatory effects. Pimecrolimus selectively inhibits the production and release of cytokines and inflammatory mediators from T-lymphocytes and mast cells. Pimecrolimus specifically binds to cytosolic receptor macrophilin-12 and inhibits calcium-dependent phosphatase – calcineurin.
Inhibition of calcineurin leads to suppression of T-lymphocyte proliferation and prevents transcription and production of early cytokines such as interleukin-2, interferon-γ, interleukin-4, interleukin-5, interleukin-10, tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor in T helper types 1 and 2.
Pimecrolimus and tacrolimus equally suppress the secondary immune response in isolated skin T-helper cell colonies obtained from patients with atopic dermatitis. In vitro pimecrolimus also prevents antigen/IgE-mediated release of cytokines and inflammatory mediators from mast cells.
Pimecrolimus does not affect the growth of keratinocytes, fibroblasts and endothelial cells and, unlike corticosteroids, has a selective effect on cells of the immune system and does not cause disturbances in function, viability, differentiation processes, maturation of mouse Langerhans cells and human monocytic dendritic cells. The drug does not affect the differentiation of “naive” T-lymphocytes into T-effector cells under the action of Langerhans cells and dendritic cells, which is one of the main mechanisms of the specific immune response.
High anti-inflammatory activity of pimecrolimus after its local and systemic application was demonstrated on experimental models of skin inflammation. When applied topically on experimental models of allergic contact dermatitis (ACD) pimecrolimus is comparable in effectiveness with highly active corticosteroids: clobetasol – 17-propionate and fluticasone, inhibits the inflammatory response in response to skin irritants without causing changes in skin consistency, thickening and atrophy.
In addition, pimecrolimus effectively reduces skin inflammation, itching and the severity of histopathological changes when used topically and orally in experimental models of acute AKD. When applied topically, the degree of skin penetration of tacrolimus and pimecrolimus is equally good. However, the ability of pimecrolimus to penetrate the skin is less than that of tacrolimus and glucocorticosteroids. Thus, pimecrolimus has a selective action on the skin.
The uniqueness of the mechanism of action of pimecrolimus is the combination of selective anti-inflammatory action on the skin with a minor effect on the systemic immune response.
Pimecrolimus effectively decreases itching and skin inflammation (erythema, infiltration, exoriations and lichenization) when used for 6 weeks in children from 3 months to 17 years old.
With long-term use for 12 months pimecrolimus effectively reduces the frequency of sudden ACD exacerbations without causing atrophy, irritation and increased skin hypersensitivity and without having phototoxic or photosensitizing effect.
Indications
Indications
Atopic dermatitis (eczema).
The drug is indicated for short-term and long-term treatment of atopic dermatitis in adults, adolescents and children (from 3 months).
Pharmacological effect
Pharmacological effect
Pimecrolimus is a lipophilic derivative of the macrolactam ascomycin with anti-inflammatory activity that selectively inhibits the production and release of cytokines and inflammatory mediators from T lymphocytes and mast cells. Pimecrolimus specifically binds to the cytosolic receptor macrophilin-12 and inhibits the calcium-dependent phosphatase calcineurin. Inhibition of calcineurin leads to the suppression of T-lymphocyte proliferation and prevents the transcription and production of early cytokines such as interleukin-2, interferon-γ, interleukin-4, interleukin-5, interleukin-10, tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor in T-helper cells 1 and 2. Pimecrolimus suppresses the secondary immune response in isolated skin T helper cell colonies obtained from patients with atopic dermatitis. In vitro, pimecrolimus also prevents antigen/IgE-mediated release of cytokines and inflammatory mediators from mast cells. Pimecrolimus does not affect the growth of keratinocytes, fibroblasts and endothelial cells and, unlike corticosteroids, has a selective effect on cells of the immune system and does not cause dysfunction, viability, differentiation processes, maturation of Langerhans cells in mice and dendritic cells of monocytic origin in humans. The drug does not affect the differentiation of “naïve” T-lymphocytes into T-effector cells under the influence of Langerhans cells and dendritic cells, which is one of the main mechanisms of a specific immune response.
When applied externally in experimental models of allergic contact dermatitis (ACD), pimecrolimus is comparable in effectiveness to highly active corticosteroids: clobetasol – 17-propionate and fluticasone.
When used externally, the ability of pimecrolimus to penetrate the skin is less than that of glucocorticosteroids, which makes the drug very low in its absorption into the systemic circulation. Thus, pimecrolimus has a skin-selective pharmacological profile in contrast to that of glucocorticosteroids.
When administered for 6 weeks to children 3 months to 17 years of age, pimecrolimus was effective in reducing pruritus and skin inflammation (erythema, infiltration, excoriation, and lichenification) during the first week of treatment in 44% of children and adolescents and 70% of infants. When using pimecrolimus for 12 months, a decrease in the frequency of exacerbations of ACD was observed. Tolerability studies have shown that Elidel is not a contact allergen and does not have phototoxic, photosensitizing or cumulative irritant effects.
Absorption in adults
The concentration of pimecrolimus in the blood was determined in 12 adult patients with atopic dermatitis affecting 15-59% of the body surface area who were treated with Elidel 2 times a day for 3 weeks. In 77.5% of observations, the concentration of pimecrolimus in the blood was below 0.5 ng/ml, and in 99.8% – below 1 ng/ml. The maximum blood concentration of pimecrolimus recorded in one patient was 1.4 ng/ml.
In 98% of 40 adult patients with initial lesions of 14-62% of the body surface area after 1 year of treatment with Elidel, pimecrolimus blood concentrations were less than 0.5 ng/ml. The maximum concentration of 0.8 ng/ml was recorded at the 6th week of treatment in two patients. None of the patients showed an increase in the concentration of the drug in the blood over 12 months of treatment.
In 8 adult patients with pimecrolimus blood levels above the minimum detectable concentration, the area under the pharmacokinetic curve AUC (0-12h) ranged from 2.5 to 11.4 ng/h/ml.
Absorption in children
Systemic exposure to pimecrolimus was studied in 58 children aged from
3 months to 14 years with lesions of 10-92% of the body surface area, treated with Elidel 2 times a day for 3 weeks (five children were treated for 1 year as needed).
Pimecrolimus blood concentrations were consistently low, regardless of application area and duration of treatment, and were in the same range as in adult patients. In 97% of cases, pimecrolimus blood concentrations were below 2 ng/ml, and in 60% – below 0.5 ng/ml. The maximum concentration of pimecrolimus recorded in two patients aged 8 months and 14 years was 2.0 ng/ml.
In infants (aged 3 to 23 months), the maximum blood concentration of pimecrolimus was 2.6 ng/ml (in one patient). In five children treated for 1 year, blood concentrations of pimecrolimus were consistently low (maximum value was 1.94 ng/ml in 1 patient). Throughout the entire treatment period, an increase in drug concentrations was not observed in any of the patients.
Distribution
In in vitro studies, the binding of pimecrolimus to plasma proteins (mainly various lipoproteins) was 99.6%.
Metabolism
Since the concentrations of pimecrolimus in the blood are very low when applied externally, it is not possible to study the metabolism of pimecrolimus after external use.
Studies with single oral administration of radiolabeled pimecrolimus to healthy subjects have shown that unchanged pimecrolimus is the major drug component in the blood associated with the active substance, and that there are many minor drug metabolites of moderate polarity that appear to be products of O-demethylation and oxidation reactions. In in vitro studies, pimecrolimus was not metabolized in human skin.
Removal
The radioactive tracer associated with the active substance was excreted primarily through the intestines (78.4%) and only to a small extent (2.5%) by the kidneys.
Special instructions
Special instructions
In clinical studies with the use of Elidel, the development of lymphadenopathy was noted in 0.9% of patients (14 out of 1544). Typically, lymphadenopathy was caused by infectious diseases and resolved after a course of appropriate antibiotic therapy. If lymphadenopathy develops while using Elidel, the patient should undergo an examination to determine the cause of this symptom. If the etiology of lymphadenopathy is unknown or the patient has acute mononucleosis inflammation, treatment with the drug should be discontinued.
During treatment with Elidel, patients should avoid excessive ultraviolet exposure, including tanning beds, PUVA therapy, UVA or UVA exposure.
If Elidel comes into contact with the eyes, mucous membranes of the mouth or nasal cavity, immediately remove the cream and rinse the eyes and mucous membranes with running water.
Active ingredient
Active ingredient
Pimecrolimus
Composition
Composition
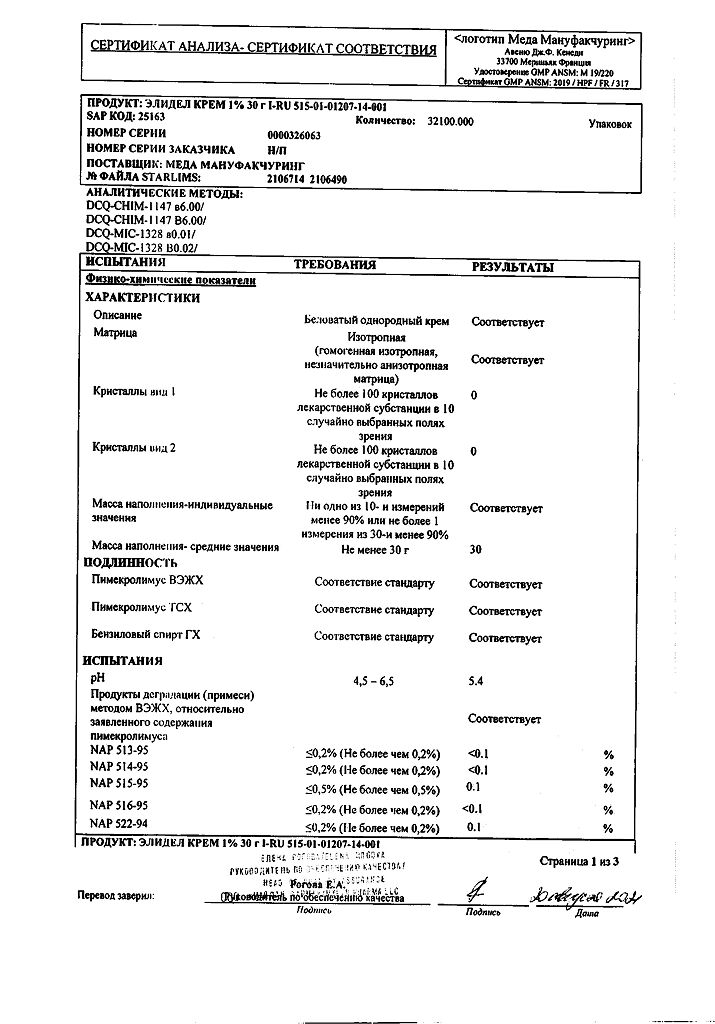
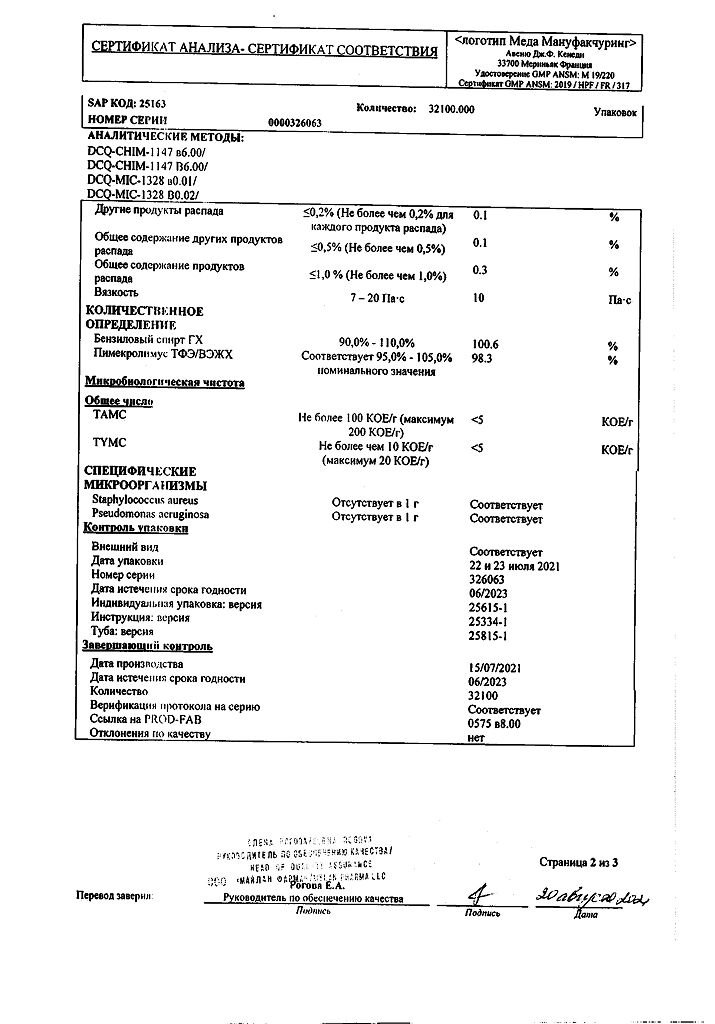
1 g of cream contains:
active ingredient: pimecrolimus – 10 mg;
excipients: sodium hydroxide 0.20 mg, anhydrous citric acid 0.50 mg, benzyl alcohol 10.00 mg, sodium cetostearyl sulfate 10.00 mg, mono and diglycerides 20.00 mg, cetyl alcohol 40.00 mg, stearyl alcohol 40.00 mg, propylene glycol 50.00 mg, oleyl alcohol 100.00 mg, medium chain triglycerides 150.00 mg, purified water 569.30 mg.
Pregnancy
Pregnancy
Pregnancy. Elidel should not be used during pregnancy, as there is insufficient data on the use of Elidel in pregnant women. Lactation. There are no data on the content of pimecrolimus in breast milk in lactating women. Caution should be exercised when prescribing Elidel to nursing women. Elidel should not be applied to the breast area.
There is no data on the effect of Elidel cream on fertility in men and women.
Contraindications
Contraindications
Hypersensitivity to pimecrolimus, other macrolactams or any excipients in the drug.
Children under 3 months of age (since the safety and effectiveness of Elidel cream in children under 3 months of age has not been studied).
Side Effects
Side Effects
The most common adverse events – reactions at the site of application of the drug – were observed in 19% of patients treated with Elidel and in 16% of patients in the control group. These reactions mainly developed early in treatment, were minor/moderate and short-lived.
The incidence of side effects is determined as follows:
Very common – ≥1/10, Common – ≥1/100<1/10, Uncommon - ≥1/1000<1/100, Rare - ≥1/10,000<1/1000, Very rare - <1/10,000, Frequency not established (cannot be determined based on available data).
Reactions at the site of application of the drug:
Very common: burning sensation.
Common: irritation, itching, redness.
Uncommon: rash, paresthesia, peeling, dryness, pain, swelling.
From the immune system:
Very rarely – anaphylactic reactions, including severe ones.
Metabolism:
Rarely, alcohol intolerance (shortly after drinking alcohol, facial redness (“hot flashes”), rash, burning sensation, itching, swelling developed).
From the skin and subcutaneous tissues:
Often – skin infections (folliculitis);
Not often – boil, impetigo, infection caused by the herpes simplex virus, herpes zoster, herpetic eczema, skin papilloma, aggravation of the underlying disease;
Rarely, allergic reactions (rash, urticaria, angioedema); changes in skin color (hypo- or hyperpigmentation).
Infections and infestations:
Uncommon: Molluscum contagiosum.
Cases of malignant neoplasms, including skin lymphoma and other types of lymphoma, as well as skin cancer, have been reported with the use of Elidel cream.
However, eight epidemiological studies did not show an increased risk of malignancy associated with topical pimecrolimus exposure in any population.
Interaction
Interaction
Systematic studies of the interaction of Elidel with other drugs have not been conducted. Given that the systemic absorption of pimecrolimus is negligible, its interaction with systemic medications is unlikely.
Data obtained to date indicate that Elidel can be used simultaneously with antibacterial and antihistamine drugs, as well as glucocorticosteroids (for oral, intranasal and inhalation administration)
When using Elidel cream in infants from 3 to 12 months and children aged 2 years and older, the drug did not affect the effectiveness of vaccination.
It is not recommended to apply cream to the area where the vaccine was administered until local manifestations of the post-vaccination reaction have completely disappeared.
Incompatibility
Since compatibility studies have not been conducted, it is not recommended to use the drug in combination with other external agents.
Overdose
Overdose
There have been no cases of overdose or accidental ingestion of Elidel.
Specifications
Specifications
According to the recipe.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25ºС, do not freeze. The drug should be stored out of the reach of children.
Shelf life
Shelf life
2 years. The drug should not be used after the expiration date. After first opening, use within 1 year.
Manufacturer
Manufacturer
Meda Manufacturing, France
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | Store at temperatures below 25°C. Do not freeze. Keep the drug out of reach of children. |
| Manufacturer | Meda Manufacturing, France |
| Medication form | exterior cream |
| Brand | Meda Manufacturing |
Related products
Buy Elidel, cream 1% 30 g with delivery to USA, UK, Europe and over 120 other countries.