No products in the cart.
Effex Sildenafil, 50 mg 6 pcs
€5.92 €5.27
Description Pharmacokinetics:
Sildenafil is a potent selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
The physiological mechanism of erection is based on the release of nitric oxide (NO) in the corpora cavernosa during sexual stimulation. This in turn increases cGMP levels, which relaxes the smooth muscle tissue in the corpora cavernosa and increases blood flow in the corpora cavernosa.
Sildenafil does not have a direct relaxing effect on the isolated corpora cavernosa but enhances the relaxing effect of nitric oxide by inhibiting FDE5, which is responsible for the breakdown of cGMP in the corpora cavernosa.
The pharmacological effect is achieved only in the presence of sexual stimulation.
In vitro studies have shown that sildenafil is selective against FDE5. Its activity against other known isoenzymes is much lower: FDE6 by a factor of 10, FDE1 by a factor of more than 80, FDE2, FDE4, FDE7-11 by a factor of more than 700. Sildenafil is 4,000 times more active against FDE5 as compared to FDEA, which is important because FDEA is one of the key enzymes regulating myocardial contractility.
A prerequisite for the effectiveness of sildenafil is sexual stimulation.
The use of sildenafil in doses up to 100 mg resulted in a mild, transient decrease in blood pressure. The hypotensive effect is associated with the vasodilatory effect of sildenafil associated with an increase in cGMP levels in vascular smooth muscle cells.
In some patients, 1 hour after taking the drug at a dose of 100 mg, the Farnsworth-Munsel 100 test revealed a mild and transient impairment of the ability to distinguish shades of color (blue/green). After two hours, color perception was restored. Color vision impairment is caused by inhibition of FDE6, which is involved in the process of light transmission in the retina. Sildenafil does not affect visual acuity, contrast perception, electroretinogram values, intraocular pressure, or pupil diameter.
Intake
It is rapidly absorbed after oral administration. Maximum plasma concentration is reached within 30-120 minutes (60 minutes on average) when taken orally on an empty stomach. Bioavailability varies from 25 to 63%. In combination with fatty food it reduces absorption speed: Cmax decreases on the average by 29%, and time of reaching of maximum concentration (Tmax) is increased by 60 min, but absorption degree does not change significantly (area under the pharmacokinetic curve of concentration – time (AUC) is reduced by 11%).
Distribution
The volume of distribution of sildenafil in the equilibrium state averages 105 l. Binding to plasma proteins of sildenafil and its main circulating N-demethyl metabolite is approximately 96% and is independent of the total drug concentration. Less than 0.0002% of the dose (188 ng on average) is detected in semen 90 min after sildenafil administration.
Metabolism
Sildenafil is metabolized mainly in the liver by microsomal cytochrome P450 isoenzymes: CYP3A4 (major pathway) and CYP2C9 (minor pathway). The main circulating metabolite formed as a result of N-demethylation of sildenafil undergoes further metabolism. Selectivity of this metabolite on FDE is comparable with that of sildenafil, and its activity against FDE5 in vitro is about 50% of sildenafil activity. Plasma concentration of the metabolite is about 40% of sildenafil concentration. N-demethyl metabolite undergoes further metabolism; its final elimination half-life is about 4 hours.
Elimination
The total clearance of sildenafil is 41 l/hour, and the final half-life of sildenafil is 3-5 hours. After oral administration, sildenafil is excreted as metabolites mainly by the intestine (about 80% of the oral dose) and, to a lesser extent, by the kidneys (about 13% of the oral dose).
Pharmacokinetics in special patient groups
Elderly patients
In healthy elderly patients (65 years and older), sildenafil clearance is reduced and free sildenafil plasma concentrations are about 40% higher than in younger patients (18-45 years). Age has no clinically significant effect on the incidence of side effects.
Renal dysfunction
In mild (creatinine clearance (CK) 50-80 ml/min) and moderate (CK 30-49 ml/min) renal impairment, the pharmacokinetics of sildenafil after a single oral dose of 50 mg is unchanged. In severe renal impairment (CKR <30 ml/min) sildenafil clearance is decreased, resulting in approximately two-fold increase of area under pharmacokinetic curve of concentration-time AUC (100%) and Cmax (88%) compared to those in normal renal function patients of the same age group.
Hepatic disorders
In patients with cirrhosis (Child-Pugh stages A and B), sildenafil clearance is decreased, resulting in increased AUC (84%) AND Cmax (47%) compared to those in normal liver function in patients in the same age group. Pharmacokinetics of sildenafil in patients with severe hepatic impairment (Child-Pugh stage C) have not been studied.
.
Indications
Indications
Treatment of erectile dysfunction, characterized by the inability to achieve or maintain penile erection sufficient for satisfactory sexual intercourse.
EFFECT Sildenafil is effective only with sexual stimulation.
Pharmacological effect
Pharmacological effect
Sildenafil is a powerful selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
The physiological mechanism of erection is the release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. This, in turn, leads to an increase in cGMP levels, resulting in relaxation of the smooth muscle tissue of the corpus cavernosum and increased blood flow in the corpus cavernosum.
Sildenafil does not have a direct relaxing effect on the isolated corpus cavernosum, but enhances the relaxing effect of nitric oxide, causing inhibition of PDE5, which is responsible for the breakdown of cGMP in the corpus cavernosum.
The pharmacological effect is achieved only in the presence of sexual stimulation.
In vitro studies have shown that sildenafil is selective for PDE5. Its activity in relation to other known isoenzymes is much lower: PDE6 – 10 times, PDE1 – more than 80 times, PDE2, PDE4, PDE7-11 – more than 700 times. Sildenafil is 4000 times more active against PDE5 compared to PDEZ, which is of great importance since PDEZ is one of the key enzymes in the regulation of myocardial contractility.
A prerequisite for the effectiveness of sildenafil is sexual stimulation.
The use of sildenafil in doses up to 100 mg led to a slight short-term decrease in blood pressure. The hypotensive effect is associated with the vasodilating effect of sildenafil, which is associated with an increase in the level of cGMP in vascular smooth muscle cells.
In some patients, 1 hour after taking the drug at a dose of 100 mg, the Farnsworth-Munsell 100 test revealed a mild and transient impairment in the ability to distinguish shades of color (blue/green). After two hours, color perception was restored. Color vision impairment is caused by inhibition of PDE6, which is involved in light transmission in the retina. Sildenafil does not affect visual acuity, contrast perception, electroretinogram readings, intraocular pressure or pupil diameter.
Pharmacokinetics:
Suction
After oral administration, it is quickly absorbed. The maximum concentration in blood plasma is achieved within 30-120 minutes (average 60 minutes) when taken orally on an empty stomach. Bioavailability varies from 25 to 63%. When taken in combination with fatty foods, the rate of absorption decreases: Cmax decreases by an average of 29%, and the time to reach maximum concentration (Tmax) increases by 60 minutes, but the degree of absorption does not significantly change (the area under the concentration-time pharmacokinetic curve (AUC) decreases by 11%).
Distribution
The volume of distribution of sildenafil at steady state averages 105 liters. The plasma protein binding of sildenafil and its main circulating N-demethyl metabolite is approximately 96% and is independent of the total drug concentration. Less than 0.0002% of the dose (average 188 ng) was found in semen 90 minutes after taking sildenafil.
Metabolism
Sildenafil is metabolized mainly in the liver under the influence of microsomal cytochrome P450 isoenzymes: CYP3A4 (major pathway) and CYP2C9 (minor pathway). The main circulating metabolite, resulting from N-demethylation of sildenafil, undergoes further metabolism. The selectivity of this metabolite for PDE is comparable to that of sildenafil, and its activity against PDE5 in vitro is about 50% of the activity of sildenafil. The concentration of the metabolite in the blood plasma is about 40% of the concentration of sildenafil. The N-demethyl metabolite undergoes further metabolism; its terminal half-life is approximately 4 hours.
Removal
The total clearance of sildenafil is 41 l/hour, and the terminal half-life is 3-5 hours. After oral administration, sildenafil is excreted as metabolites, mainly by the intestines (approximately 80% of the oral dose) and, to a lesser extent, by the kidneys (approximately 13% of the oral dose).
Pharmacokinetics in special groups of patients
Elderly patients
In healthy elderly patients (65 years and older), the clearance of sildenafil is reduced, and the concentration of free sildenafil in plasma is approximately 40% higher than in young patients (18-45 years). Age does not have a clinically significant effect on the incidence of side effects.
Renal dysfunction
With mild (creatinine clearance (CL) 50-80 ml/min) and moderate (CL 30-49 ml/min) degrees of renal failure, the pharmacokinetics of sildenafil after a single oral dose of 50 mg does not change. In severe renal failure (creatinine clearance < 30 ml/min), the clearance of sildenafil is reduced, which leads to an approximately twofold increase in the area under the pharmacokinetic concentration-time curve (AUC (100%) and Cmax (88%) compared to those with normal renal function in patients of the same age group.
Liver dysfunction
In patients with liver cirrhosis (stages A and B according to the Child-Pugh classification), the clearance of sildenafil is reduced, which leads to an increase in AUC (84%) and Cmax (47%) compared to those with normal liver function in patients of the same age group. The pharmacokinetics of sildenafil in patients with severe liver dysfunction (Child-Pugh stage C) has not been studied.
Special instructions
Special instructions
To diagnose erectile dysfunction, determine its possible causes and select adequate treatment, it is necessary to obtain a complete medical history and conduct a thorough physical examination.
Erectile dysfunction treatments should be used with caution in patients with anatomical deformation of the penis (angulation, cavernous fibrosis, Peyronie’s disease), or in patients with risk factors for priapism (sickle cell anemia, multiple myeloma, leukemia, thrombocythemia).
During post-marketing studies, cases of prolonged erection and priapism have been reported. If an erection persists for more than 4 hours, you should immediately seek medical help. If treatment for priapism is not carried out immediately, it can lead to damage to the tissue of the penis and irreversible loss of potency.
Medicines intended to treat erectile dysfunction should not be prescribed to men for whom sexual activity is undesirable.
Sexual activity poses a certain risk in the presence of heart disease, so before starting any therapy for erectile dysfunction, the doctor should refer the patient for an examination of the condition of the cardiovascular system. Sexual activity is undesirable in patients with heart failure, unstable angina, stroke or myocardial infarction in the last 6 months, life-threatening arrhythmias, arterial hypertension (BP > 170/100 mm Hg) or hypotension (BP < 90/50 mm Hg).
Clinical studies showed no difference in the incidence of myocardial infarction (1.1 per 100 people per year) or the incidence of cardiovascular death (0.3 per 100 people per year) in patients receiving sildenafil compared with patients receiving placebo.
Cardiovascular complications
During post-marketing use of sildenafil for the treatment of erectile dysfunction, adverse events such as serious cardiovascular events (including myocardial infarction, unstable angina, sudden cardiac death, ventricular arrhythmia, hemorrhagic stroke, transient ischemic attack, hypertension and hypotension) were reported, which were temporarily associated with the use of sildenafil. Most of these patients, but not all of them, had risk factors for cardiovascular complications.
Many of these adverse events occurred shortly after sexual activity, and some of them occurred after taking sildenafil without subsequent sexual activity. It is not possible to establish a direct connection between the observed adverse events and these or other factors.
Hypotension
Sildenafil has a systemic vasodilating effect, leading to a transient decrease in blood pressure, which is not a clinically significant phenomenon and does not lead to any consequences in the majority of Nazis. However, before prescribing sildenafil, the physician should carefully assess the risk of possible unwanted manifestations of the vasodilating effect in patients with relevant diseases, especially against the background of sexual activity.
Increased susceptibility to vasodilators is observed in patients with obstruction of the left ventricular outflow tract (aortic stenosis, hypertrophic obstructive cardiomyopathy), as well as with the rare syndrome of multiple system atrophy, manifested by severe dysregulation of blood pressure from the autonomic nervous system.
Since the combined use of sildenafil and α-blockers may lead to symptomatic hypotension in some sensitive patients, sildenafil should be administered with caution to patients taking α-blockers. To minimize the risk of postural hypotension in patients taking α-blockers, sildenafil should be started only after hemodynamic stability has been achieved in these patients. The advisability of reducing the initial dose of sildenafil should also be considered. The physician should inform patients about what actions to take if symptoms of postural hypotension occur.
Visual impairment
In rare cases, non-arteritic anterior ischemic optic neuropathy (NAION), a rare disease and cause of vision loss or reduction, has been reported during post-marketing use of all PDE5 inhibitors, including sildenafil. Most of these patients had risk factors including decreased papilledema/disc ratio (“congestive disc”), age over 50 years, diabetes mellitus, hypertension, coronary artery disease, hyperlipidemia, and smoking.
An observational study assessed whether recent use of the PDE5 inhibitor class of drugs was associated with acute onset of NPINSID. Results indicate an approximately 2-fold increase in the risk of NPINSID within 5 half-lives of PDE5 inhibitor use. According to the published literature, the annual incidence of NPINSID is 2.5-11.8 cases per 100,000 men aged ≥ 50 years in the general population.
In case of sudden loss of vision, patients should be advised to stop sildenafil therapy and consult a doctor immediately. Individuals who have already had a case of NPINSID are at increased risk of recurrent NPINSID. Therefore, the physician should discuss this risk with such patients, as well as discuss with them the potential for adverse effects from PDE5 inhibitors. PDE5 inhibitors, including sildenafil, should be used with caution in such patients and only in situations where the expected benefit outweighs the risk.
A small number of patients with hereditary retinitis pigmentosa have genetically determined dysfunction of retinal phosphodyseterases. There is no information on the safety of sildenafil in patients with retinitis pigmentosa, so the drug should be used with caution (see section “With caution”).
Hearing impairment
Some post-marketing and clinical studies have reported cases of sudden deterioration or loss of hearing associated with the use of all PDE5 inhibitors, including sildenafil, the majority of these patients had risk factors for sudden deterioration or loss of hearing. A cause-and-effect relationship between the use of PDE5 inhibitors and sudden hearing loss or deterioration has not been established. If there is a sudden deterioration in hearing or hearing loss while taking sildenafil, you should consult your doctor immediately.
Bleeding
Sildenafil enhances the antiplatelet effect of sodium nitroprusside, a nitric oxide donor, on human platelets in vitro. There are no data on the safety of sildenafil in patients with a tendency to bleeding or exacerbation of gastric and duodenal ulcers, so sildenafil should be used with caution in these patients.
The incidence of epistaxis in patients with pulmonary hypertension associated with diffuse connective tissue diseases was higher (sildenafil 12.9%, placebo 0%) than in patients with primary pulmonary hypertension (sildenafil 3.0%, placebo 2.4%). Patients receiving sildenafil in combination with a vitamin K antagonist had a higher incidence of epistaxis (8.8%) than patients not receiving a vitamin K antagonist (1.7%).
Use in conjunction with other treatments for erectile dysfunction
The safety and effectiveness of sildenafil in combination with other PDE5 inhibitors or other drugs for the treatment of pulmonary hypertension containing sildenafil (for example, Revatio®) or other drugs for the treatment of erectile dysfunction have not been studied, and therefore the use of such combinations is not recommended.
Impact on the ability to drive vehicles. Wed and fur.:
Since when taking sildenafil, it is possible to develop dizziness, decrease in blood pressure, develop chromatopsia, blurred vision, etc. side effects, caution should be exercised when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions. You should also be careful about the individual effect of the drug in these situations, especially at the beginning of treatment and when changing the dosage regimen.
Active ingredient
Active ingredient
Sildenafil
Composition
Composition
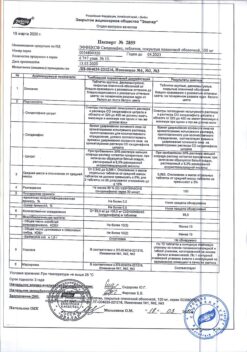
Sildenafil citrate (in terms of sildenafil), 1 tablet (50 mg) – 70.25 mg (50.00 mg).
Contraindications
Contraindications
Hypersensitivity to sildenafil or any other component of the drug.
Use in patients receiving continuous or intermittent nitric oxide donors, organic nitrates or nitrites in any form, since EFFEX Sildenafil enhances the hypotensive effect of nitrates).
The safety and effectiveness of EFFEX Sildenafil when used in combination with other drugs for the treatment of erectile dysfunction have not been studied, therefore the use of such combinations is not recommended.
Combined use with ritonavir.
Liver dysfunction.
Chronic renal failure of severe severity.
Severe heart failure, unstable angina, stroke or myocardial infarction within the last 6 months, life-threatening arrhythmias, arterial hypertension (BP > 170/100 mm Hg) or hypotension (BP < 90/50 mm Hg).
According to its registered indication, EFFEX Sildenafil is not intended for use in children under 18 years of age.
According to its registered indication, EFFEX Sildenafil is not intended for use in women.
With caution:
Anatomical deformation of the penis (angulation, cavernous fibrosis or Peyronie’s disease).
Diseases predisposing to the development of priapism (sickle cell anemia, multiple myeloma, leukemia, thrombocythemia).
Diseases accompanied by bleeding.
Exacerbation of peptic ulcer of the stomach and duodenum.
Hereditary retinitis pigmentosa.
Side Effects
Side Effects
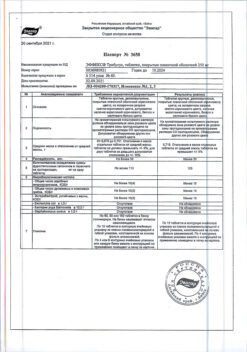
The most common side effects were headache and flushing.
Typically, the side effects of the drug EFFEX Sildenafil are mild or moderate and are transient.
Fixed-dose studies have shown that the incidence of some adverse events increases with increasing dose.
From the immune system: rarely – hypersensitivity reactions (including skin rash), allergic reactions.
From the organ of vision: often – blurred vision, blurred vision, cyanopsia; uncommon – eye pain, photophobia, photopsia, chromatopsia, redness of the eyes/scleral injections, changes in the brightness of light perception, mydriasis, conjunctivitis, hemorrhage in the eye tissue, cataracts, disruption of the lacrimal apparatus; rarely – swelling of the eyelids and adjacent tissues, a feeling of dryness in the eyes, the presence of rainbow circles in the field of view around the light source, increased eye fatigue, seeing objects in yellow (xanthopsia), seeing objects in red (erythropsia), conjunctival hyperemia, irritation of the mucous membrane of the eyes, discomfort in the eyes; frequency unknown – non-arteritic anterior ischemic optic neuropathy (NAINO), retinal vein occlusion, visual field defect, diplopia*, temporary loss of vision or decreased visual acuity, increased intraocular pressure, retinal edema, retinal vascular disease, vitreous detachment/vitreal traction.
On the part of the hearing organ: uncommon – sudden decrease or loss of hearing, tinnitus, ear pain.
From the cardiovascular system: often – “hot flashes”; uncommon – tachycardia, palpitations, decreased blood pressure, increased heart rate, unstable angina, atrioventricular block, myocardial ischemia, cerebral vascular thrombosis, cardiac arrest, heart failure, abnormal electrocardiogram readings, cardiomyopathy; rarely – atrial fibrillation.
From the blood and lymphatic system: infrequently – anemia, leukopenia.
From the side of metabolism and nutrition: infrequently – a feeling of thirst, edema, gout, uncompensated diabetes mellitus, hyperglycemia, peripheral edema, hyperuricemia, hypoglycemia, hypernatremia.
From the respiratory system: often – nasal congestion; uncommon – nosebleeds, rhinitis, asthma, dyspnea, laryngitis, pharyngitis, sinusitis, bronchitis, increased volume of sputum, increased cough; rarely – a feeling of tightness in the throat, dryness of the nasal mucosa, swelling of the nasal mucosa.
From the gastrointestinal tract: often – nausea, dyspepsia; uncommon gastroesophageal reflux disease, vomiting, abdominal pain, dry oral mucosa, glossitis, gingivitis, colitis, dysphagia, gastritis, gastroenteritis, esophagitis, stomatitis, abnormal liver function tests, rectal bleeding; rarely – hyposthesia of the oral mucosa.
From the musculoskeletal system: often – back pain; uncommon – myalgia, pain in the limbs, arthritis, arthrosis, tendon rupture, tenosynovitis, bone pain, myasthenia gravis, synovitis.
From the genitourinary system: infrequently – cystitis, nocturia, breast enlargement, urinary incontinence, hematuria, ejaculation disorders, genital swelling, anorgasmia, hematospermia, damage to penile tissue; rarely – prolonged erection and/or priapism.
From the central and peripheral nervous system: very often – headache; often – dizziness; uncommon – drowsiness, migraine, ataxia, hypertonicity, neuralgia, neuropathy, paresthesia, tremor, vertigo, symptoms of depression, insomnia, unusual dreams, increased reflexes, kinesthesia; rarely – convulsions*, repeated convulsions*, fainting.
From the skin and subcutaneous tissues: uncommon – skin rash, urticaria, herpes simplex, itching, increased sweating, skin ulceration, contact dermatitis, exfoliative dermatitis; frequency unknown – Stevens-Johnson syndrome, toxic epidermal necrolysis.
Other: infrequently – feeling of heat, swelling of the face, photosensitivity reaction, shock, asthenia, increased fatigue, pain of various localizations, chills, accidental falls, pain in the chest, accidental injuries; rarely – irritability.
*Side effects identified during post-marketing studies.
Cardiovascular complications
During the post-marketing use of sildenafil for the treatment of erectile dysfunction, adverse events such as severe cardiovascular complications (including myocardial infarction, unstable angina, sudden cardiac death, ventricular arrhythmia, hemorrhagic stroke, transient ischemic attack, hypertension and hypotension), which were temporarily associated with the use of sildenafil, were reported, the majority these patients, but not all of them, had risk factors for cardiovascular complications.
Many of these adverse events occurred shortly after sexual activity, and some of them occurred after taking sildenafil without subsequent sexual activity. It is not possible to establish a direct connection between the observed adverse events and these or other factors.
Visual impairment
In rare cases, non-arteritic anterior ischemic neuropathy (NAIAN), a rare disease and cause of decreased or loss of vision, has been reported during post-marketing use of all PDE5 inhibitors, including sildenafil. Most of these patients had risk factors including decreased papilledema/disc ratio (“congestive disc”), age over 50 years, diabetes mellitus, hypertension, coronary artery disease, hyperlipidemia, and smoking. An observational study assessed whether recent use of the PDE5 inhibitor class of drugs was associated with acute onset of NPINSID.
Results indicate an approximately 2-fold increase in the risk of NPINSID within 5 half-lives of PDE5 inhibitor use. Published literature estimates the annual incidence of NPINSID to be 2.5–11.8 cases per 100,000 men aged >50 years in the general population.
In case of sudden loss of vision, patients should be advised to stop sildenafil therapy and consult a doctor immediately. Individuals who have already had a case of NPINSID are at increased risk of recurrent NPINSID. Therefore, the physician should discuss this risk with such patients, as well as discuss with them the potential for adverse effects from PDE5 inhibitors. PDE5 inhibitors, including sildenafil, should be used with caution in such patients and only in situations where the expected benefit outweighs the risk.
Interaction
Interaction
The metabolism of sildenafil occurs mainly under the influence of the cytochrome CYP3A4 (main pathway) and CYP2C9 isoenzymes, therefore inhibitors of these isoenzymes can reduce the clearance of sildenafil, and inducers, accordingly, increase the clearance of sildenafil.
A decrease in the clearance of sildenafil was noted with simultaneous use of inhibitors of the cytochrome CYP3A4 isoenzyme (ketoconazole, erythromycin, cimetidine).
Cimetidine (800 mg), a nonspecific inhibitor of the cytochrome CYP3A4 isoenzyme, when taken together with sildenafil (50 mg), causes an increase in plasma sildenafil concentrations by 56%.
A single dose of 100 mg of sildenafil together with erythromycin (500 mg/day 2 times a day for 5 days), a moderate inhibitor of the cytochrome CYP3A4 isoenzyme, while achieving a constant concentration of erythromycin in the blood, leads to an increase in the AUC of sildenafil by 182%.
When co-administered with sildenafil (100 mg once) and saquinavir (1200 mg/day 3 times a day), an inhibitor of HIV protease and the cytochrome CYP3A4 isoenzyme, while achieving a constant concentration of saquinavir in the blood, the Cmax of sildenafil increased by 140%, and the AUC increased by 210%. Sildenafil has no effect on the pharmacokinetics of saquinavir.
Stronger inhibitors of the cytochrome CYP3A4 isoenzyme, such as ketoconazole and itraconazole, may cause more severe changes in the pharmacokinetics of sildenafil.
The simultaneous use of sildenafil (100 mg once) and ritonavir (500 mg 2 times a day), an HIV protease inhibitor and a strong cytochrome P450 inhibitor, while achieving a constant concentration of ritonavir in the blood leads to an increase in sildenafil Cmax by 300% (4 times), and AUC by 1000% (11 times). After 24 hours, the concentration of sildenafil in the blood plasma is about 200 ng/ml (after a single use of sildenafil alone – 5 ng/ml).
If sildenafil is used in recommended doses by patients simultaneously receiving strong inhibitors of the cytochrome CYP3A4 isoenzyme, then the Cmax of free sildenafil does not exceed 200 nM, and the drug is well tolerated.
A single dose of an antacid (magnesium hydroxide/aluminum hydroxide) does not affect the bioavailability of sildenafil.
In studies in healthy volunteers, co-administration of the endothelin receptor antagonist bosentan (an inducer of CYP3A4 (moderate), CYP2C9 and possibly CYP2C19) at steady-state concentration (125 mg twice daily) and sildenafil at steady-state concentration (80 mg three times daily) resulted in a decrease in sildenafil AUC and Cmax by 62.6% and 52.4% accordingly.
Sildenafil increased the AUC and Cmax of bosentan by 49.8% and 42%, respectively. It is assumed that the simultaneous use of sildenafil with strong inducers of the CYP3A4 isoenzyme, such as rifampicin, may lead to a large decrease in the concentration of sildenafil in the blood plasma.
Inhibitors of the cytochrome CYP2C9 isoenzyme (tolbutamide, warfarin), the cytochrome CYP2D6 isoenzyme (selective serotonin reuptake inhibitors, tricyclic antidepressants), thiazide and thiazide-like diuretics, ACE inhibitors and calcium antagonists do not affect the pharmacokinetics of sildenafil.
Azithromycin (500 mg/day for 3 days) has no effect on the AUC, Cmax, Tmax, elimination rate constant and T1/2 of sildenafil or its main circulating metabolite.
Effect of sildenafil on other drugs
Sildenafil is a weak inhibitor of cytochrome P450 isoenzymes – 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 (IC50> 150 µmol). When sildenafil is taken at recommended doses, its Cmax is approximately 1 µmol, so it is unlikely that sildenafil could affect the clearance of substrates of these isoenzymes.
Sildenafil enhances the hypotensive effect of nitrates both with long-term use of the latter and when they are prescribed for acute indications. In this regard, the use of sildenafil in combination with nitrates or nitric oxide donors is contraindicated.
When taking the α-blocker doxazosin (4 mg and 8 mg) and sildenafil (50 mg and 100 mg) simultaneously in patients with benign prostatic hyperplasia with stable hemodynamics, the average additional decrease in systolic/diatonic blood pressure in the supine position was 9/5 mm Hg. Art. and 8/4 mm Hg. Art. respectively, and in a standing position – 11/4 mm Hg. Art. and 4/5 mm Hg. Art. respectively.
Rare cases of symptomatic postural hypotension, manifested in the form of dizziness (without fainting), have been reported in such patients. In selected sensitive patients receiving α-blockers, concomitant use of sildenafil may lead to symptomatic hypotension.
There were no signs of significant interaction with tolbutamide (250 mg) or warfarin (40 mg), which are metabolized by the cytochrome CYP2C9 isoenzyme.
Sildenafil (100 mg) does not affect the pharmacokinetics of HIV protease inhibitors, saquinavir and ritonavir, which are substrates of the cytochrome CYP3A4 isoenzyme, at their constant blood levels.
Co-administration of sildenafil at steady state (80 mg three times daily) leads to an increase in the AUC and Cmax of bosentan (125 mg twice daily) by 49.8% and 12%, respectively.
Sildenafil (50 mg) does not cause an additional increase in bleeding time when taking acetylsalicylic acid (150 mg).
Sildenafil (50 mg) does not enhance the hypotensive effect of alcohol in healthy volunteers with a maximum blood alcohol concentration of 0.08% (80 mg/dL) on average.
In patients with arterial hypertension, no signs of interaction between sildenafil (100 mg) and amlodipine were detected. The average additional decrease in blood pressure in the supine position is 8 mm Hg. Art. (systolic) and 7 mm Hg. Art. (diastolic). The use of sildenafil in combination with antihypertensive drugs does not lead to additional side effects.
Overdose
Overdose
When using the drug EFFEX Sildenafil in doses exceeding the recommended ones, adverse events were similar to those noted above, but usually occurred more often.
Treatment is symptomatic. Hemodialysis does not accelerate the elimination of the drug, since sildenafil binds tightly to plasma proteins and is not excreted by the kidneys.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Evalar CJSC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C. Store out of the reach of children. |
| Manufacturer | Evalar, Russia |
| Medication form | pills |
| Brand | Evalar |
Other forms…
Related products
Buy Effex Sildenafil, 50 mg 6 pcs with delivery to USA, UK, Europe and over 120 other countries.