No products in the cart.
Description
Antitumor antibiotic of the anthracycline series isolated from the culture of Streptomyces peucetius var. caesius.
Indications
Indications
Breast cancer, small cell lung cancer, mesothelioma, esophageal cancer, stomach cancer, primary hepatocellular cancer, insulinoma, carcinoid, malignant tumors of the head and neck, thyroid cancer, malignant thymoma, ovarian cancer, testicular germ cell tumors, prostate cancer, bladder cancer (treatment and prevention of relapses after surgery), endometrial cancer, cervical cancer, uterine sarcoma, soft tissue sarcoma, Ewing sarcoma, osteogenic sarcoma, rhabdomyosarcoma, neuroblastoma, Wilms tumor, Kaposi’s sarcoma in AIDS, acute lymphoblastic leukemia, acute myeloblastic leukemia, chronic lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, multiple myeloma.
Pharmacological effect
Pharmacological effect
Antitumor antibiotic of the anthracycline series, isolated from the culture of Streptomyces peucetius var. caesius.
Special instructions
Special instructions
Use with caution in patients with heart disease (including a history), chicken pox (including recent or after contact with sick people), herpes zoster, other acute infectious diseases, gout or nephrolithiasis (including a history), as well as in patients who have undergone mediastinal radiation therapy or are simultaneously receiving cyclophosphamide.
During the treatment period, regular monitoring of the peripheral blood picture, laboratory parameters of liver function, ECG and ultrasound of the heart (with determination of the left ventricular ejection fraction) is necessary. If the leukocyte count is less than 3500/μl and the platelet count is less than 100,000/μl, the dose of doxorubicin is reduced by 50%.
Cases of the development of severe, life-threatening arrhythmias have been described immediately or within several hours after doxorubicin administration.
It is not recommended to vaccinate patients and their families.
Doxorubicin may cause urine to turn red for 1 to 2 days after administration.
Experimental studies have established the carcinogenic and mutagenic effects of doxorubicin.
Active ingredient
Active ingredient
Doxorubicin
Composition
Composition
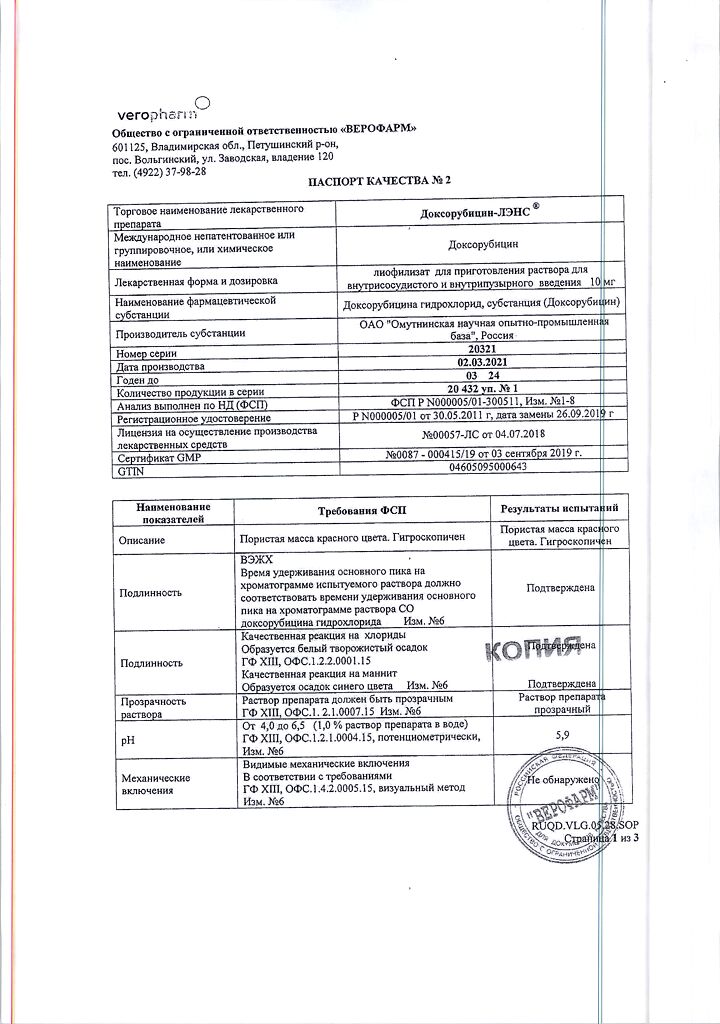
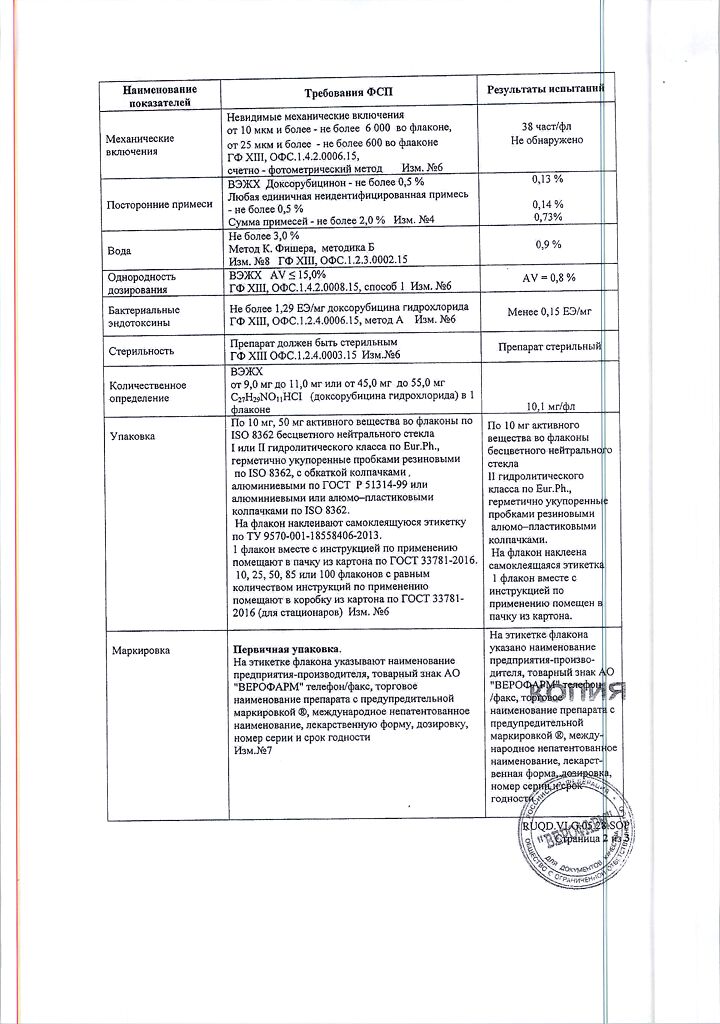
1 bottle contains:
Active substance:
Pregnancy
Pregnancy
Doxorubicin is contraindicated for use during pregnancy. If necessary, use during lactation should stop breastfeeding.
Women of childbearing potential should use reliable methods of contraception when using doxorubicin.
Experimental studies have established the teratogenic and embryotoxic effects of doxorubicin.
Contraindications
Contraindications
– Hypersensitivity to doxorubicin or other components of the drug, as well as to other anthracyclines and anthracenediones. – Pregnancy and lactation period.
– Intravenous administration is contraindicated in: severe myelosuppression, severe liver failure, severe heart failure and arrhythmias, recent myocardial infarction, previous therapy with other anthracyclines or anthracenediones in maximum total doses, acute viral infections (including chicken pox and herpes zoster).
– Injection into the bladder is contraindicated for: invasive tumors with penetration into the bladder wall, urinary tract infections, inflammation of the bladder, hematuria.
With caution Patients with risk factors for cardiotoxicity; patients who have previously received intensive chemotherapy, children, elderly patients, obese patients, patients with tumor infiltration of the bone marrow (a reduction in the starting 3 doses or an increase in the intervals between doses may be required); use as part of combination antitumor therapy, as well as in combination with radiation or other antitumor therapy; patients with impaired liver function; patients with gastric and duodenal ulcers (with intra-arterial administration of doxorubicin).
Side Effects
Side Effects
From the hematopoietic organs: dose-dependent, reversible leukopenia and neutropenia. Thrombocytopenia and anemia may also develop. Leukopenia usually reaches its lowest value 10-14 days after administration of the drug; restoration of the blood picture is usually observed on day 21.
From the cardiovascular system: When therapy with anthracyclines there is a risk of developing cardiotoxicity – early (i.e. acute) or late (delayed). The cardiotoxic effect usually manifests itself within 1-6 months. after starting treatment. The manifestation of early (acute) cardiotoxicity of doxorubicin is primarily sinus tachycardia and/or ECG abnormalities (nonspecific changes in ST-T waves). Tachyarrhythmias (including ventricular tachycardia), ventricular extrasystole, as well as bradycardia, atrioventricular block and His bundle block may also be observed. The occurrence of these phenomena is not always a prognostic factor for the development of subsequent delayed cardiotoxicity, they are rarely clinically significant, and do not require discontinuation of doxorubicin therapy.
Late (delayed) myocardial damage is manifested by a decrease in left ventricular ejection fraction without clinical symptoms and/or symptoms of congestive heart failure (CHF) (shortness of breath, pulmonary edema, peripheral edema, cardiomegaly and hepatomegaly, oliguria, ascites, exudative pleurisy, gallop rhythm). Subacute effects (pericarditis/myocarditis) may also occur. The most severe form of anthracycline-induced cardiomyopathy is life-threatening CHF, which presents cumulative dose-limiting toxicity. Phlebitis, thrombophlebitis, thromboembolic complications, including pulmonary embolism (in some cases fatal).
From the digestive system: anorexia, nausea/vomiting, mucositis/stomatitis, hyperpigmentation of the oral mucosa, esophagitis, abdominal pain, gastric erosion, bleeding from the gastrointestinal tract, diarrhea, colitis, dehydration, changes in transaminase levels.
From the urinary system: urine turns red within 1-2 days after administration of doxorubicin.
From the senses: conjunctivitis, keratitis, lacrimation.
From the reproductive system: amenorrhea (at the end of therapy, ovulation is restored, but premature menopause may occur); oligospermia, azoospermia (in some cases, the sperm count is restored to normal levels; this can happen several years after the end of therapy).
From the skin and skin appendages: in most cases, reversible complete alopecia develops. Hair regrowth usually begins 2-3 months after stopping the drug. Hyperpigmentation of the skin and nails, photosensitivity, urticaria, rash, and itching may also occur. Some patients who had previously undergone radiation therapy after doxorubicin administration (usually after 4-7 days) experienced hypersensitivity of irritated skin, the appearance of erythema with the formation of blisters, swelling, severe pain, and moist epidermitis in areas corresponding to the radiation fields.
Allergic reactions: skin rash, dermatitis, urticaria, hyperemia of the skin of the palms and soles, bronchospasm, anaphylaxis (rare).
Local reactions: erythematous striations are often detected along the vein into which the infusion was made, then local phlebitis or thrombophlebitis may occur. Phlebosclerosis may also develop, especially if doxorubicin is repeatedly injected into a small vein. If the drug gets into surrounding tissues, local pain, severe inflammation of the subcutaneous tissue and tissue necrosis may occur.
With intra-arterial administration: in addition to systemic toxicity, gastric and duodenal ulcers may occur (probably due to reflux of drugs into the gastric artery); narrowing of the bile ducts due to drug-induced sclerosing cholangitis, widespread necrosis of the perfused tissue.
With intravesical administration: cystitis, bladder constriction.
Other: malaise, asthenia, fever, chills, a “rush” of blood to the skin of the face (with rapid intravenous administration), hyperuricemia or nephropathy associated with increased formation of uric acid, the development of acute lymphocytic or myelocytic leukemia, the addition of secondary infections, sepsis/septicemia, red staining of urine.
Interaction
Interaction
Doxorubicin may increase the toxicity of other antineoplastic agents, especially myelotoxicity and gastrointestinal toxicity. When doxorubicin is used concomitantly with other cytotoxic drugs that have potential cardiotoxicity (for example, fluorouracil and/or cyclophosphamide), as well as with blockers of “slow” calcium channels (for example, verapamil), more careful monitoring of cardiac function is required throughout the course of therapy. Against the background of doxorubicin, it is possible to increase the phenomena of hemorrhagic cystitis caused by cyclophosphamide and increase the hepatotoxicity of mercaptopurine.
Overdose
Overdose
An acute overdose of doxorubicin can lead to severe myelosuppression (mainly leukopenia and thrombocytopenia), toxic effects on the gastrointestinal tract, and cause acute cardiac damage.
Recommendations for use
Recommendations for use
Doxorubicin can be used both as monotherapy and in combination with other anticancer drugs in various doses depending on the treatment regimen. When individually selecting a dose, one should be guided by data from specialized literature.
Intravenous administration:
– as monotherapy, the recommended dose per cycle is 60-75 mg/m2 every three weeks. Usually the drug is administered once during the cycle; however, the cyclic dose can be divided into several administrations (for example, administered during the first three consecutive days, or on the first and eighth days of the cycle), with cycles repeated every 3-4 weeks.
– to reduce the toxic effect of doxorubicin, especially cardiotoxicity, a weekly dosage regimen of 10-20 mg/m2 is used;
– in combination with other antitumor drugs, doxorubicin is prescribed in a cyclic dose of 30-60 mg/m2 every 3-4 weeks.
Liver dysfunction. In patients with hyperbilirubinemia, the dose of doxorubicin should be reduced according to the concentration of total bilirubin:
– by 50% when the concentration of bilirubin in the blood serum is 12-30 mg/l;
– by 75% when the concentration of bilirubin in the blood serum is above 30 mg/l.
Other special patient groups. It is recommended to prescribe lower doses or increase the intervals between cycles in patients who have previously received massive antitumor therapy, in children, in elderly patients, in obese patients (if body weight is more than 130% of ideal, there is a decrease in the systemic clearance of doxorubicin), as well as in patients with tumor infiltration of the bone marrow. Repeated administration of the drug is possible only after the disappearance of all signs of toxicity (especially gastrointestinal and hematological).
Rules for preparing the solution and administration: doxorubicin lyophilisate is dissolved in 0.9% sodium chloride solution or water for injection. The resulting solution with the required amount of doxorubicin is further diluted with 0.9% solution of 4 sodium chloride or water for injection to a concentration of no more than 1 mg/1 ml. The drug is administered intravenously slowly (over 3-5 minutes) into the injection port of the intravenous infusion system, during a rapid infusion of 5% dextrose solution or 0.9% sodium chloride solution. Before injection, you must ensure that the needle or catheter is positioned exactly in the vein. Avoid injection into small veins and veins above the joints; Care should be taken not to perform venipuncture and subsequent administration of doxorubicin on the extremities where there are disturbances in venous and lymphatic outflow.
The total dose of doxorubicin should not exceed 550 mg/m2. In patients who have previously received radiation therapy to the mediastinal/pericardial region or who have taken other cardiotoxic drugs, if it is necessary to increase the total dose of doxorubicin to more than 450 mg/m2, the drug should be administered under strict monitoring of cardiac function. Before administration, the required dose of the drug should be dissolved in 0.9% sodium chloride solution or water for injection to a concentration of 2 mg/ml. The drug is administered intravenously in a slow stream.
Introduction into the bladder.
The recommended dose for intravesical administration is 30-50 mg per instillation, with intervals between administrations from 1 week to 1 month, depending on the goals of therapy – treatment or prevention. The recommended concentration of the solution is 1 mg/1 ml of water for injection or 0.9% sodium chloride solution. After completion of instillation, to ensure uniform effect of the drug on the bladder mucosa, patients should turn from side to side every fifteen minutes. As a rule, the drug should remain in the bladder for 1-2 hours.
At the end of instillation, the patient must empty the bladder. To prevent excessive dilution of the drug by urine, patients should be warned to withhold fluids for 12 hours before instillation. Systemic absorption of doxorubicin when instilled into the bladder is very low.
In case of manifestations of local toxic effects (chemical cystitis, which can be manifested by dysuria, polyuria, nocturia, painful urination, hematuria, discomfort in the bladder area, necrosis of the bladder wall), the dose intended for repeated instillation should be dissolved in 50-100 ml of 0.9% sodium chloride solution. Particular attention should be paid to problems 5 associated with catheterization (for example, urethral obstruction caused by massive intravesical tumors).
Intra-arterial administration.
In patients with hepatocellular cancer, to provide intense local exposure while reducing the overall toxic effect, doxorubicin can be administered intra-arterially into the main hepatic artery at a dose of 30-150 mg/m2 with an interval of 3 weeks to 3 months. Higher doses should be used only in cases where extracorporeal excretion of the drug is simultaneously carried out. Since this method is potentially dangerous, and widespread tissue necrosis may occur when used, intra-arterial administration should only be performed by physicians who are fluent in this technique.
Storage conditions
Storage conditions
At a temperature not exceeding 8 °C, out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Lance Farm, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | Keep out of reach of children at a temperature not exceeding 8 oC. |
| Manufacturer | Lance Farm, Russia |
| Medication form | lyophilizate |
| Brand | Lance Farm |
Related products
Buy Doxorubicin, lyophilizate 10 mg with delivery to USA, UK, Europe and over 120 other countries.