No products in the cart.
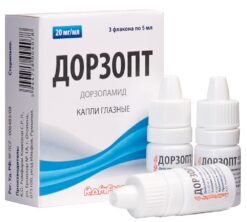
Dorzopt, eye drops 2% 5 ml
€8.38 €7.33
Description
A local carbohydrase inhibitor, an anti-glaucoma drug. Reduces intraocular fluid secretion by slowing down the formation of hydrocarbonate with subsequent weakening of sodium and water transport. It does not cause accommodation spasm, miosis, hemeralopia.
Pharmacokinetics
It penetrates the eye mainly through the cornea (to a lesser extent through the sclera or limbus). Systemic absorption is low. After entering the blood, it quickly penetrates into red blood cells containing a significant amount of carboanhydrase II.
The binding to plasma proteins is 33%. It is transformed into N-desethylated metabolite, which is less active with respect to carboanhydrase II, but is able to block carboanhydrase I. With long-term use, it cumulates in erythrocytes.
Extracted by the kidneys unchanged and as metabolites. After withdrawal, the fast phase of excretion is followed by a slow phase caused by gradual release of the drug from the red blood cells, with a half-life (T 1/2 ) of about 4 months.
Indications
Indications
Ocular hypertension, primary open-angle glaucoma, pseudoexfoliative glaucoma, secondary glaucoma (without blocking the anterior chamber angle); as an additional therapy to beta-blockers, as monotherapy when beta-blockers are ineffective or there are contraindications to them.
Treatment of glaucoma in children from the 1st week of life – as monotherapy or in addition to treatment with beta-blockers.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: Antiglaucoma agent – carbonic anhydrase inhibitor
Pharmacological action
Antiglaucoma agent, selective inhibitor of human carbonic anhydrase II. Inhibition of carbonic anhydrase in the ciliary processes of the eye leads to a decrease in the secretion of aqueous humor, resulting in a decrease in intraocular pressure (IOP). Dorzolamide has minimal or virtually no effect on heart rate or blood pressure.
Pharmacokinetics
When applied topically, dorzolamide penetrates the systemic circulation. During a course of use, due to selective binding to carbonic anhydrase II, it accumulates in erythrocytes. In this case, very low concentrations of unchanged active substance are determined in the blood plasma. Dorzolamide is metabolized to form a single N-desethyl metabolite, which also accumulates in red blood cells. The binding of dorzolamide to plasma proteins is about 33%. It is excreted primarily in the urine in the form of unchanged substance and metabolite. After the end of the administration of dorzolamide, the process of leaching from erythrocytes is nonlinear: at the beginning there is a rapid decrease in the concentration of the active substance, then the elimination slows down, with T1/2 being 4 months.
Special instructions
Special instructions
Use with caution in patients with a history of recurrent corneal erosions and/or surgical interventions affecting the integrity of the eyeball; the likelihood of corneal edema increases.
Elderly patients may have increased sensitivity to dorzolamide.
If allergic reactions develop, you should stop using dorzolamide.
Impact on the ability to drive vehicles and machinery
Since dorzolamide can cause dizziness and nausea, during treatment, potentially hazardous activities associated with the need for concentration and increased speed of psychomotor reactions should be avoided.
Active ingredient
Active ingredient
Dorzolamide
Composition
Composition
Active substance:
dorzolamide
Excipients: hydroxyethylcellulose – 1 mg, mannitol – 20 mg, citric acid monohydrate – 4 mg, sodium hydroxide – 2.426 mg, benzalkonium chloride – 0.1 mg, purified water – up to 1 ml.
Pregnancy
Pregnancy
The use of dorzolamide is contraindicated during pregnancy and breastfeeding.
Contraindications
Contraindications
Hypersensitivity to dorzolamide, severe renal failure, hyperchloremic acidosis, pregnancy, breastfeeding, children under 1 week of age.
Side Effects
Side Effects
From the organ of vision: very often – burning, pain; often – superficial punctate keratitis, lacrimation, conjunctivitis, inflammation of the eyelids, itching, irritation and swelling of the eyelids, blurred vision; uncommon – iridocyclitis; rarely – redness of the eyes, pain, hyperkeratosis of the eyelids, transient myopia, disappearing after discontinuation of the drug, corneal edema, hypotony of the eye, retinal detachment in patients after surgical interventions to restore the outflow of intraocular fluid.
From the nervous system: often – headache; rarely – dizziness, paresthesia.
From the respiratory system: rarely – nosebleeds, sore throat; frequency unknown – shortness of breath.
From the digestive system: often – nausea, bitterness in the mouth; rarely – dry mouth.
From the cardiovascular system: frequency unknown – palpitation
From the skin and subcutaneous tissues: rarely – contact dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis.
From the urinary tract: rarely – urolithiasis.
From the immune system: rarely – allergic reactions from the eyelids, symptoms of systemic allergic reactions – incl. angioedema, urticaria, itching, rash, difficulty breathing, less often – bronchospasm.
General reactions: often – asthenia, fatigue.
Interaction
Interaction
There is a potential for additive effects to the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic anhydrase inhibitor and dorzolamide.
Increased toxicity may occur when taking acetylsalicylic acid in high doses.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years.
Once opened, the bottle should be stored for no more than 4 weeks.
Manufacturer
Manufacturer
K.O.Rompharm Company S.R.L., Romania
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | C.O.Rompharm Company S.R.L., Romania |
| Medication form | eye drops |
| Brand | C.O.Rompharm Company S.R.L. |
Other forms…
Related products
Buy Dorzopt, eye drops 2% 5 ml with delivery to USA, UK, Europe and over 120 other countries.