No products in the cart.
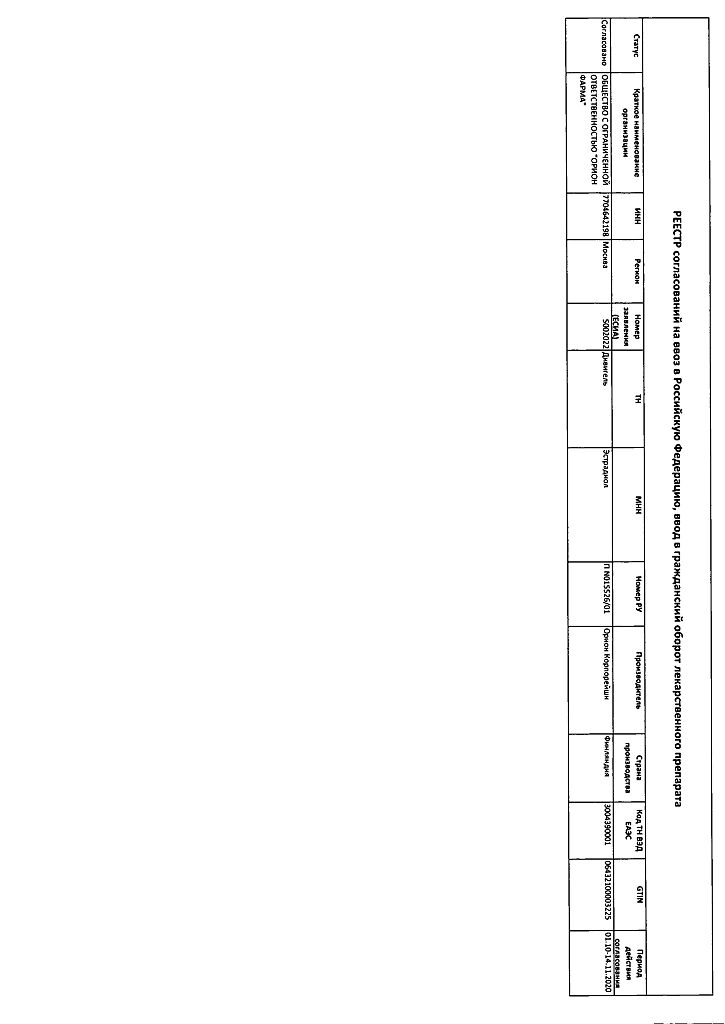
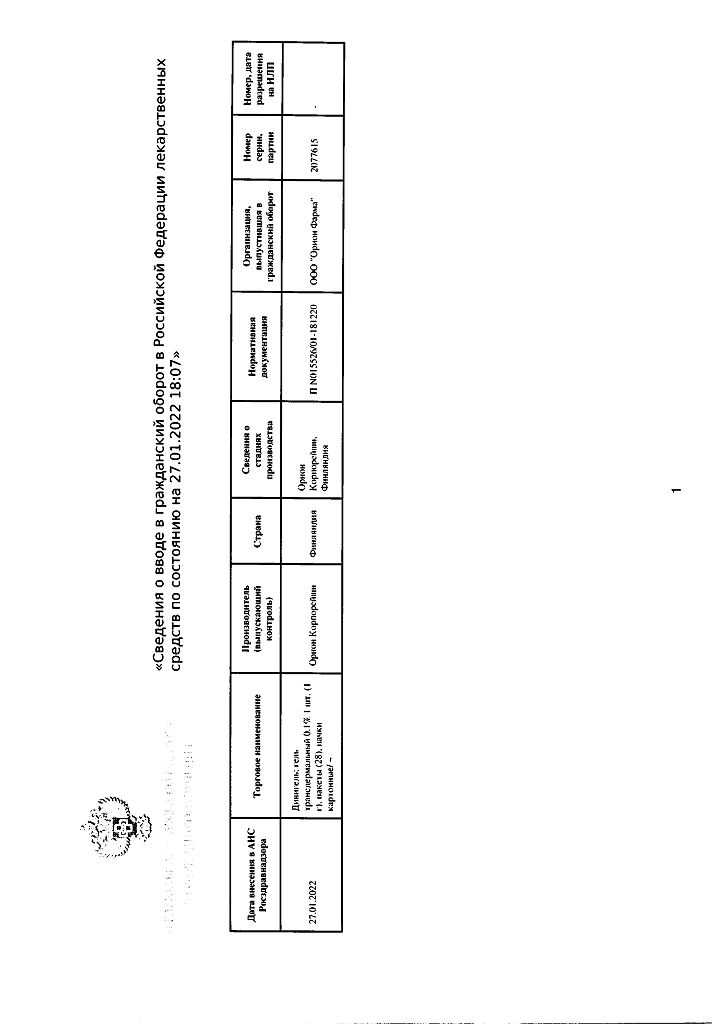
Divigel, transdermal gel 0.1% 1 g bags 28 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Divigel is an estrogenic drug for external use. The active ingredient, synthetic 17β-estradiol, is chemically and biologically identical to endogenous human estradiol (produced in women from their first menstruation until menopause), which is produced by the ovaries.
In the cells of the organs that are targeted by the hormones, estrogens form a complex with specific receptors (found in various organs – the uterus, vagina, urethra, breast, liver, hypothalamus, pituitary gland); the receptor-ligand complex interacts with estrogen effector elements of the genome and specific intracellular proteins, inducing the synthesis of i-RNA, proteins and the release of cytokines and growth factors.
It has a feminizing effect on the body. It stimulates development of uterus, fallopian tubes, vagina, stroma and ducts of mammary glands, pigmentation of nipples and genitalia, formation of female secondary sexual characteristics, growth and closure of epiphyses of long tubular bones.
Promotes timely rejection of endometrium and regular bleeding, in high concentrations causes endometrial hyperplasia, inhibits lactation, inhibits bone resorption, stimulates synthesis of several transport proteins (thyroxine-binding globulin, transcortin, transferrin, protein binding sex hormones), fibrinogen. It has procoagulant effect, induces the synthesis in the liver of vitamin K-dependent clotting factors (II, VII, IX, X), reduces the concentration of antithrombin III.
Enhances concentrations of thyroxine, iron and copper in the blood. Has anti-atherosclerotic effect, increases HDL, reduces LDL and cholesterol (triglyceride levels increase).
Modulates sensitivity of receptors to progesterone and sympathetic regulation of smooth muscle tone, stimulates the transition of intravascular fluid in tissues and causes compensatory retention of sodium and water. At high doses it prevents the degradation of endogenous catecholamines by competing for active COMT receptors.
After menopause only a small amount of estradiol is produced in the body (from estrone, which is in the liver and in adipose tissue). The decrease in estradiol produced in the ovaries is accompanied in many women by vasodilatory and thermoregulatory instability (flushing of the skin of the face), sleep disorders, and progressive atrophy of the genitourinary system.
Osteoporosis (mainly of the spine) develops due to estrogen deficiency. After ingestion, more estradiol is metabolized in the lumen (microflora) and intestinal wall, as well as in the liver (which leads to non-physiologically high plasma concentrations of estrone and estrone sulfate cumulation during long-term therapy) before it enters the bloodstream.
The consequences of the accumulation of these metabolites in the body over a long period of time have not yet been elucidated. It is known that oral estrogen use causes increased synthesis of proteins (including renin), which leads to increased BP.
Indications
Indications
Hormone replacement therapy for symptoms of estrogen deficiency; treatment of climacteric syndrome associated with natural or artificial menopause that develops as a result of surgical intervention.
Pharmacological effect
Pharmacological effect
Divigel is an estrogenic drug for external use. The active ingredient is synthetic 17β-estradiol, chemically and biologically identical to endogenous human estradiol (formed in the body of women from the first menstruation until menopause) produced by the ovaries.
In the cells of the organs to which the action of hormones is directed, estrogens form a complex with specific receptors (found in various organs – in the uterus, vagina, urethra, mammary gland, liver, hypothalamus, pituitary gland); the receptor-ligand complex interacts with estrogen effector elements of the genome and specific intracellular proteins that induce the synthesis of mRNA, proteins and the release of cytokines and growth factors.
Has a feminizing effect on the body. Stimulates the development of the uterus, fallopian tubes, vagina, stroma and ducts of the mammary glands, pigmentation in the area of the nipples and genitals, the formation of secondary sexual characteristics of the female type, the growth and closure of the epiphyses of long tubular bones.
Promotes timely endometrial rejection and regular bleeding, in high concentrations causes endometrial hyperplasia, suppresses lactation, inhibits bone resorption, stimulates the synthesis of a number of transport proteins (thyroxine-binding globulin, transcortin, transferrin, sex hormone-binding protein), fibrinogen. It has a procoagulant effect, induces the synthesis of vitamin K-dependent blood coagulation factors (II, VII, IX, X) in the liver, and reduces the concentration of antithrombin III.
Increases blood concentrations of thyroxine, iron, copper. It has an anti-atherosclerotic effect, increases HDL content, reduces LDL and cholesterol (triglyceride levels increase).
Modulates the sensitivity of receptors to progesterone and sympathetic regulation of smooth muscle tone, stimulates the transition of intravascular fluid into tissues and causes compensatory retention of sodium and water. In high doses, it prevents the degradation of endogenous catecholamines by competing for active COMT receptors.
After menopause, only a small amount of estradiol is produced in the body (from estrone found in the liver and adipose tissue). A decrease in the content of estradiol produced in the ovaries is accompanied in many women by vasomotor and thermoregulatory instability (flushes to the facial skin), sleep disorders, as well as progressive atrophy of the genitourinary system.
Osteoporosis (mainly of the spine) develops due to estrogen deficiency. After oral administration, larger amounts of estradiol are metabolized in the lumen (microflora) and intestinal wall, as well as in the liver, before entering the bloodstream (leading to non-physiologically high concentrations of estrone in plasma, and with long-term therapy, to the accumulation of estrone and estrone sulfate).
The consequences of the accumulation of these metabolites in the body over a long period of time have not yet been clarified. It is known that oral administration of estrogens causes an increase in protein synthesis (including renin), which leads to an increase in blood pressure.
Special instructions
Special instructions
A complete personal and family history should be obtained before starting or restarting hormone replacement therapy. A medical examination should be conducted to identify possible contraindications and observe the necessary precautions when using the drug (including the pelvic organs and mammary glands).
During the treatment process, it is recommended to conduct periodic examinations; the frequency and set of methods included in it are determined for each specific case individually. Studies, including mammography, should be carried out in accordance with accepted standards and taking into account individual clinical characteristics in each individual case.
During HRT, all benefits and risks of therapy should be carefully assessed.
The patient should be under constant medical supervision in case of any of the following diseases or conditions that were previously observed and/or aggravated during pregnancy or previous hormonal therapy: leiomyoma (uterine fibroids), endometriosis; history of thromboembolic diseases or risk factors for their occurrence; risk factors for estrogen-dependent tumors (1st degree of heredity for breast cancer); arterial hypertension; liver dysfunction (adenoma); diabetes mellitus with or without vascular lesions; cholelithiasis; migraine and/or (severe) headache; systemic lupus erythematosus; history of endometrial hyperplasia; epilepsy; bronchial asthma; otosclerosis. It should be borne in mind that during treatment with Divigel, in rare cases, a relapse or exacerbation of the listed diseases is possible.
Therapy should be stopped immediately if contraindications are found and/or in the following situations: jaundice or deterioration of liver function; pronounced increase in blood pressure; new attacks of migraine-like headaches; pregnancy.
When taking estrogens for a long time, the risk of developing endometrial hyperplasia and carcinoma increases. To reduce the risk, it is necessary to combine estrogen therapy in women with an unoperated uterus with progesterone for at least 12 days during the treatment cycle.
If breakthrough bleeding and/or scanty spotting occurs after several months of taking Divigel, studies should be conducted to identify the causes of their occurrence. Investigations may include endometrial biopsy (to rule out endometrial malignancy).
For women with a uterus removed due to endometriosis (especially in cases of residual endometriosis), the addition of progesterone to estrogen-dependent therapy is recommended, due to premalignant or malignant transformation of endometriosis foci with estrogen stimulation.
Long-term use of HRT increases the risk of developing breast cancer. According to epidemiological studies among women aged 50 to 70 years, breast cancer is diagnosed in 45 cases out of 1000. It has been established that among women taking or recently taking HRT, the total number of additional cases of breast cancer during the corresponding period is 1-3 (on average 2) additional cases per 1000 people receiving HRT for 5 years; 3-9 (average 6) cases per 1000 people receiving HRT for 10 years and 5-20 (average 12) cases per 1000 women receiving HRT for 15 years. An increase in this risk was found mainly in women of thin or normal build. In women of full build (high susceptibility to breast cancer), HRT does not further increase the risk of developing breast cancer.
An additional risk of developing breast cancer appears with increasing duration of HRT use and returns to baseline within approximately 5 years after stopping treatment.
Combined estrogen-progestogen HRT carries a similar or higher risk compared to estrogen therapy.
In women receiving HRT, the risk of developing venous thromboembolic diseases (thrombosis of the deep veins of the lower extremities and pulmonary veins) is increased by 2-3 times compared with women who did not receive HRT. The likelihood is higher in the first year of HRT than in subsequent years.
The main risk factors for thromboembolic complications: individual or family history, severe obesity (body mass index more than 30 kg/m2), systemic lupus erythematosus.
Patients with a history of thromboembolism or recent spontaneous miscarriages should undergo additional studies to exclude a predisposition to thrombophlebitis. The use of HRT in this case should be started after a full assessment of the risk factors for the development of thrombophlebitis and the initiation of anticoagulant therapy. The risk increases with prolonged immobilization, major trauma, or major surgery. HRT should be discontinued 4-6 weeks before planned abdominal surgery or orthopedic surgery on the lower extremities. Treatment can be resumed after complete restoration of motor ability. With the development of thromboembolic symptoms (sudden chest pain, dyspnea), discontinuation of HRT may be required.
Estrogens cause fluid retention in the body. Patients with impaired renal function should be under constant medical supervision due to increased levels of estradiol and its metabolites in the blood.
Estrogens increase sensitivity to insulin and increase its excretion. For patients with diabetes mellitus, constant monitoring of blood glucose levels is indicated in the first months of HRT.
Taking estrogen increases the risk of surgically confirmed cholelithiasis.
In rare cases of a sharp increase in triglyceride levels in the blood while taking estrogen, pancreatitis may develop.
Estrogens increase levels of thyroid binding globulin, increasing the level of total circulating thyroid hormones.
Avoid contact of the gel with the mammary glands and mucous membranes of the vulva and vagina.
Impact on the ability to drive vehicles and operate machinery
Therapy with Divigel does not affect the ability to engage in potentially hazardous activities that require increased attention and speed of psychomotor reactions.
Active ingredient
Active ingredient
Estradiol
Composition
Composition
1 g of gel contains:
Active substance:
estradiol 1 mg;
Excipients:
carbomers (carbopol 974 R);
trolamine;
propylene glycol;
ethyl alcohol (96%);
purified water – up to 1.0 g.
Pregnancy
Pregnancy
Divigel is contraindicated for use during pregnancy and lactation.
Contraindications
Contraindications
breast cancer (diagnosed, suspected or history);
diagnosed or suspected estrogen-dependent malignant tumors of the ovaries, uterus, endometrium;
benign and malignant neoplasms of the genital organs (cervical and uterine cancer, uterine fibroids, vulvar cancer, ovarian cancer) in women under 60 years of age;
benign breast tumors in women under 60 years of age;
vaginal bleeding of unknown etiology and a tendency to uterine bleeding;
endometrial hyperplasia;
pituitary tumors;
diffuse connective tissue diseases;
inflammatory diseases of the female genital organs (salpingoophoritis, endometritis);
hyperestrogenic stage of menopause;
spontaneous thromboembolic diseases of the veins (including a history);
deep vein thrombosis, pulmonary embolism (including history);
thrombophlebitis and acute thrombophlebitis (including history);
congenital hyperbilirubinemia (Gilbert, Dubin-Johnson, Rotor syndromes);
liver tumors (hemangioma, liver cancer);
cerebrovascular accidents (ischemic stroke, hemorrhagic stroke);
diabetes mellitus, retinopathy, angiopathy;
sickle cell anemia;
disorders of fat metabolism;
cholestatic jaundice or severe cholestatic itching (including increased manifestations during a previous pregnancy or while taking steroid drugs);
otosclerosis (including its exacerbation during pregnancy);
hypersensitivity to estradiol and/or other components of the drug.
With caution: bronchial asthma, migraine, epilepsy, arterial hypertension, heart failure, coronary artery disease, liver and/or kidney failure, edema syndrome, endometriosis, fibrocystic mastopathy, porphyria. Experience with women over 65 years of age is limited.
Divigel should not be applied to the mammary glands, face, genital area, or to irritated areas of the skin.
Side Effects
Side Effects
From the central nervous system and peripheral nervous system: headache, migraine, dizziness, depression, chorea.
From the cardiovascular system: increased blood pressure, thrombophlebitis.
From the digestive system: nausea, vomiting, stomach colic, flatulence, pain in the epigastric region, cholestatic jaundice, cholelithiasis.
Allergic reactions: at the site of application – rash, skin irritation, skin hyperemia, contact dermatitis.
From the reproductive system: metrorrhagia, scanty bleeding, increased size of uterine leiomyoma, endometrial hyperplasia (when prescribed without combination with progesterone), endometrial carcinoma (in women with an intact uterus after menopause), ovarian sclerosis with long-term use, changes in libido.
From the endocrine system: engorgement (tension and/or enlargement) of the mammary glands, weight gain, decreased tolerance to carbohydrates.
Metabolism: sodium, calcium and water retention (edema) with prolonged use; attacks of porphyria.
Other: visual impairment (changes in corneal curvature), chloasma, melasma, vaginal candidiasis.
Interaction
Interaction
Estradiol increases the effectiveness of lipid-lowering drugs; weakens the effect of male sex hormones; hypoglycemic, diuretic, antihypertensive drugs and anticoagulants; reduces glucose tolerance (dose adjustment of hypoglycemic drugs).
The metabolism of estradiol is accelerated when taken simultaneously with barbiturates, tranquilizers (anxiolytics), opioid analgesics, anesthetics, some antiepileptic drugs (carbamazepine, phenytoin), inducers of microsomal liver enzymes; herbal preparations containing the herb St. John’s wort (St. John’s herb).
The concentration of estradiol in the blood also decreases with the simultaneous use of phenylbutazone and certain antibiotics (ampicillin, rifampicin, rifabutin) and antiviral drugs (nevirapine, efavirenz), which is associated with changes in intestinal microflora.
The effect of estradiol increases when taking folic acid and thyroid medications.
Overdose
Overdose
Symptoms: pain in the mammary glands or pelvic area, bloating, anxiety, irritability, nausea, vomiting, and in some cases, metrorrhagia.
Treatment: symptomatic therapy.
Symptoms disappear when the dose is reduced or when the drug is discontinued.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Orion Corporation, Finland
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Orion Corporation, Finland |
| Medication form | transdermal gel |
| Brand | Orion Corporation |
Related products
Buy Divigel, transdermal gel 0.1% 1 g bags 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.