No products in the cart.
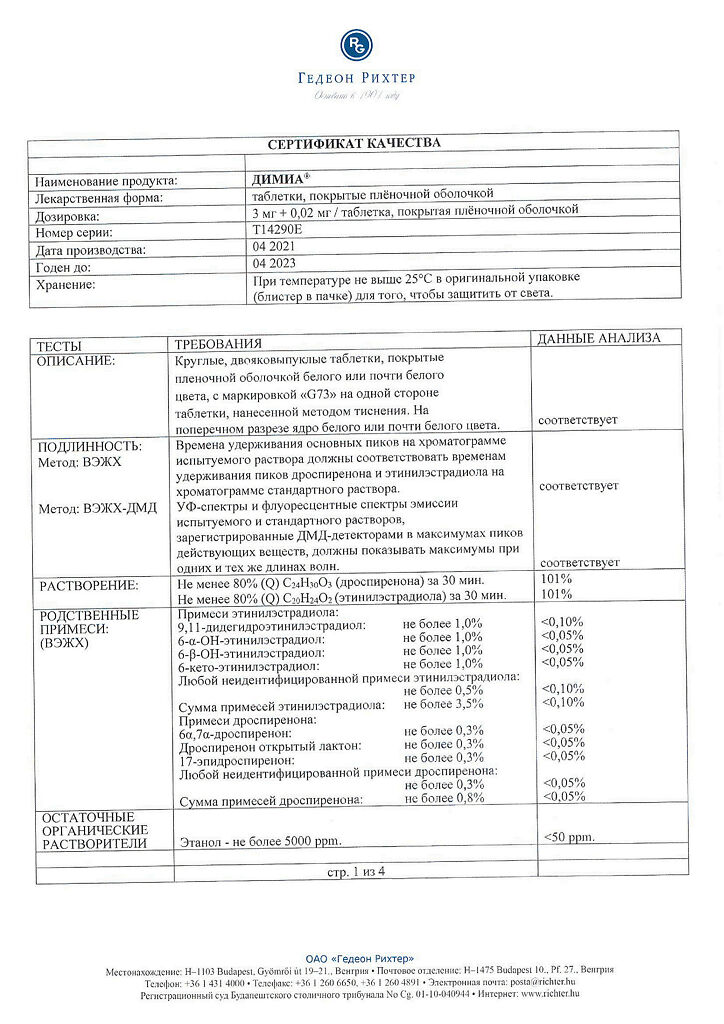
Dimia, 3 mg+0.02 mg 28 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Dimia is a combined monophasic oral contraceptive containing drospirenone and ethinylestradiol.
In its pharmacological profile drospirenone is close to natural progesterone: it has no estrogenic, glucocorticoid and antiglucocorticoid activity and is characterized by pronounced antiandrogenic and moderate anti-mineralocorticoid action.
The contraceptive effect is based on the interaction of various factors, the most important of which are inhibition of ovulation, increased cervical secretion viscosity and changes in the endometrium.
The Perl index, a measure of the frequency of pregnancy in 100 women of reproductive age during a year of contraceptive use, is less than 1.
Indications
Indications
Oral contraception.
Pharmacological effect
Pharmacological effect
Dimia is a combined monophasic oral contraceptive containing drospirenone and ethinyl estradiol.
In terms of its pharmacological profile, drospirenone is close to natural progesterone: it does not have estrogenic, glucocorticoid and antiglucocorticoid activity and is characterized by pronounced antiandrogenic and moderate antimineralocorticoid effects.
The contraceptive effect is based on the interaction of various factors, the most important of which are inhibition of ovulation, increased viscosity of cervical secretions and changes in the endometrium.
The Pearl index, an indicator reflecting the pregnancy rate in 100 women of reproductive age during a year of contraceptive use, is less than 1.
Special instructions
Special instructions
If any of the conditions/risk factors listed below exist, the benefits of taking COCs should be assessed individually for each woman and discussed with her before starting use. If an adverse event worsens or if any of these conditions or risk factors occur, the woman should contact her doctor. The doctor must decide whether to stop taking the COC.
Circulatory disorders
Taking any COC increases the risk of venous thromboembolism (VTE). The increase in the risk of VTE is most pronounced in the first year of a woman’s use of COCs.
Epidemiological studies have shown that the incidence of VTE in women without risk factors taking low-dose estrogens (<0.05 mg ethinyl estradiol) in COCs is approximately 20 cases per 100,000 woman-years (for second-generation levonorgestrel-containing COCs) or 40 cases per 100,000 woman-years (for desogestrel/gestodene-containing COCs third generation). Women who do not use COCs have an incidence of 5–10 VTE and 60 pregnancies per 100,000 woman-years. VTE is fatal in 1–2% of cases.
Data from a large, prospective, 3-arm study showed that the incidence of VTE in women with or without other risk factors for VTE who used the combination of ethinyl estradiol and drospirenone 0.03+3 mg was similar to the incidence of VTE in women who used levonorgestrel-containing oral contraceptives and other COCs. The risk of VTE when taking Dimia® has not currently been established.
Epidemiological studies have also revealed an association between COC use and an increased risk of arterial thromboembolism (myocardial infarction, transient ischemic events).
Very rarely, thrombosis of other blood vessels, such as veins and arteries of the liver, mesentery, kidney, brain or retina, has occurred in women taking oral contraceptives. There is no consensus regarding the connection of these phenomena with the use of hormonal contraceptives.
Symptoms of venous or arterial thrombotic/thromboembolic events or acute cerebrovascular accidents:
– unusual unilateral pain and/or swelling of the lower extremities;
– sudden severe pain in the chest, regardless of whether it radiates to the left arm or not;
– sudden shortness of breath;
– sudden onset of cough;
– any unusual, severe, prolonged headache;
– sudden partial or complete loss of vision;
– diplopia;
– impaired speech or aphasia;
– vertigo;
– collapse with or without partial epileptic seizures;
– weakness or very noticeable numbness that suddenly affects one side or part of the body;
– movement disorders;
– acute stomach.
Before starting to take COCs, a woman should consult a specialist. The risk of venous thromboembolic disorders when taking COCs increases:
– with increasing age;
– hereditary predisposition (VTE has ever occurred in siblings or parents at a relatively early age);
– prolonged immobilization, extended surgery, any surgery on the lower extremities or major trauma. In such situations, it is recommended to stop taking the drug (in case of planned surgery, at least 4 weeks in advance) and not to resume until two weeks have passed after complete restoration of mobility. If the drug is not stopped promptly, anticoagulant treatment should be considered;
– obesity (body mass index more than 30);
– lack of consensus on the possible role of varicose veins and superficial thrombophlebitis in the appearance or exacerbation of venous thrombosis.
The risk of arterial thromboembolic complications or acute cerebrovascular accident increases when taking COCs:
– with increasing age;
– smoking (women over 35 years of age are strongly advised to quit smoking if they want to take COCs);
– dislipoproteinemia;
– arterial hypertension;
– migraine without focal neurological symptoms;
– obesity (body mass index more than 30);
– hereditary predisposition (arterial thromboembolism ever in siblings or parents at a relatively early age). If a hereditary predisposition is possible, a woman should consult a specialist before starting to take COCs;
– damage to the heart valves;
– atrial fibrillation.
Having one major risk factor for venous disease or multiple risk factors for arterial disease may also be a contraindication. Anticoagulant therapy should also be considered. Women taking COCs should be properly instructed to inform their physician if symptoms of thrombosis are suspected. If thrombosis is suspected or confirmed, COC use should be discontinued. It is necessary to start adequate alternative contraception due to the teratogenicity of anticoagulant therapy with indirect anticoagulants – coumarin derivatives.
The increased risk of thromboembolism in the postpartum period should be taken into account.
Other medical conditions associated with adverse vascular events include diabetes mellitus, SLE, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis), and sickle cell disease.
An increase in the frequency or severity of migraine while taking COCs may be an indication for their immediate discontinuation.
Tumors
The most significant risk factor for developing cervical cancer is infection with the human papillomavirus. Some epidemiological studies have reported an increased risk of cervical cancer with long-term COC use, but there remains controversy regarding the extent to which these findings are attributable to confounding factors, such as testing for cervical cancer or the use of barrier methods of contraception.
A meta-analysis of the results of 54 epidemiological studies found a small increase in the relative risk (RR = 1.24) of developing breast cancer in women who are currently taking COCs. The risk gradually decreases over 10 years after stopping COC use. Since breast cancer rarely develops in women under 40 years of age, an increase in the number of breast cancer diagnoses in COC users has little effect on the overall likelihood of developing breast cancer. These studies did not find sufficient evidence of causality. The increased risk may result from earlier diagnosis of breast cancer in COC users, the biological effects of COCs, or a combination of both factors. Diagnosed breast cancer in women who had ever taken COCs was clinically less severe, which was due to early diagnosis of the disease.
Rarely, benign liver tumors have occurred in women taking COCs, and even more rarely, malignant liver tumors have occurred. In some cases, these tumors were life-threatening (due to intra-abdominal bleeding). This should be taken into account when making a differential diagnosis in the event of severe abdominal pain, liver enlargement, or signs of intra-abdominal bleeding.
Other
The progestogen component of the drug Dimia® is an aldosterone antagonist that retains potassium in the body. In most cases, an increase in potassium levels is not expected. However, in a clinical study in some patients with mild to moderate kidney disease who were taking potassium-sparing medications, serum potassium levels increased slightly while taking drospirenone. Therefore, it is recommended to monitor serum potassium levels during the first cycle of treatment in patients with renal failure whose serum potassium concentration was at the ULN level before treatment, and especially when taking potassium-sparing drugs simultaneously. Women with hypertriglyceridemia or a hereditary predisposition to it may have an increased risk of pancreatitis when taking COCs. Although small increases in blood pressure were observed in many women taking COCs, clinically significant increases were rare. Only in these rare cases is it reasonable to immediately stop taking COCs. If, when taking COCs in patients with concomitant arterial hypertension, blood pressure constantly increases or significantly elevated blood pressure cannot be corrected with antihypertensive drugs, taking the COC should be discontinued. After normalization of blood pressure with the help of antihypertensive drugs, COC use can be resumed.
The following diseases appeared or worsened both during pregnancy and when taking COCs: jaundice and/or itching associated with cholestasis, gallstones; porphyria; SLE; hemolytic-uremic syndrome; rheumatic chorea (Sydenham’s chorea); herpes during pregnancy; otosclerosis with hearing loss. However, evidence of their relationship with COC use is inconclusive.
In women with hereditary angioedema, exogenous estrogens may induce or worsen symptoms of edema.
Acute or chronic liver disease may be an indication to stop taking COCs until liver function tests normalize. Recurrence of cholestatic jaundice and/or pruritus associated with cholestasis, which developed during a previous pregnancy or with earlier use of sex hormones, is an indication for discontinuation of COCs.
Although COCs may affect peripheral insulin resistance and glucose tolerance, modification of the treatment regimen in patients with diabetes mellitus while taking low-hormone COCs (containing <0.05 mg ethinyl estradiol) is not indicated. However, women with diabetes should be closely monitored, especially in the early stages of taking COCs.
While taking COCs, an increase in endogenous depression, epilepsy, Crohn’s disease and ulcerative colitis was observed.
Chloasma may occur from time to time, especially in women who have a history of chloasma during pregnancy. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet light while taking COCs.
Drospirenone + ethinyl estradiol coated tablets contain 48.53 mg of lactose monohydrate, placebo tablets contain 37.26 mg of anhydrous lactose per tablet. Patients with rare hereditary diseases (such as galactose intolerance, lactase deficiency or glucose-galactose malabsorption) who are on a lactose-free diet should not take this drug.
Women who are allergic to soy lecithin may experience allergic reactions.
The effectiveness and safety of Dimia® as a contraceptive have been studied in women of reproductive age. It is assumed that in the postpubertal period up to 18 years of age, the effectiveness and safety of the drug are similar to those in women after 18 years of age. The use of the drug before menarche is not indicated.
Medical examinations
Before starting or re-using Dimia®, obtain a complete medical history (including family history) and exclude pregnancy. It is necessary to measure blood pressure and conduct a medical examination, guided by contraindications and precautions. A woman should be reminded to carefully read the instructions for use and adhere to the recommendations contained therein. The frequency and content of the survey should be based on existing practice guidelines. The frequency of medical examinations is individual for each woman, but should be carried out at least once every 6 months.
Women should be reminded that oral contraceptives do not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Reduced efficiency
The effectiveness of COCs may be reduced, for example, if you miss a dose of drospirenone + ethinyl estradiol tablets, have gastrointestinal disorders while taking drospirenone + ethinyl estradiol tablets, or take other medications at the same time.
Insufficient cycle control
As with other COCs, a woman may experience acyclic bleeding (spotting or withdrawal bleeding), especially in the first months of use. Therefore, any irregular bleeding should be assessed after a three-month adaptation period.
If acyclic bleeding recurs or begins after several regular cycles, the possibility of developing disorders of a non-hormonal nature should be taken into account and measures should be taken to exclude pregnancy or cancer, including therapeutic and diagnostic curettage of the uterine cavity. Some women do not experience withdrawal bleeding during the placebo phase. If the COC was taken in accordance with the instructions for use, then it is unlikely that the woman is pregnant. However, if the rules of dosage were violated before the first missed menstrual-like withdrawal bleeding or two bleedings were missed, pregnancy should be excluded before continuing to take the COC.
Impact on the ability to drive vehicles and machinery. Not identified.
Active ingredient
Active ingredient
Drospirenone, Ethinylestradiol
Composition
Composition
Ethinyl estradiol + drospirenone tablets
1 tablet contains:
active substances:
ethinyl estradiol 0.02 mg,
rospirenone 3 mg,
excipients:
lactose monohydrate – 48.53 mg;
corn starch – 16.6 mg;
pregelatinized corn starch – 9.6 mg;
macrogol and polyvinyl alcohol copolymer – 1.45 mg;
magnesium stearate – 0.8 mg,
film shell:
Opadry II white 85G18490 (polyvinyl alcohol – 0.88 mg, titanium dioxide – 0.403 mg, macrogol 3350 – 0.247 mg, talc – 0.4 mg, soy lecithin – 0.07 mg) – 2 mg
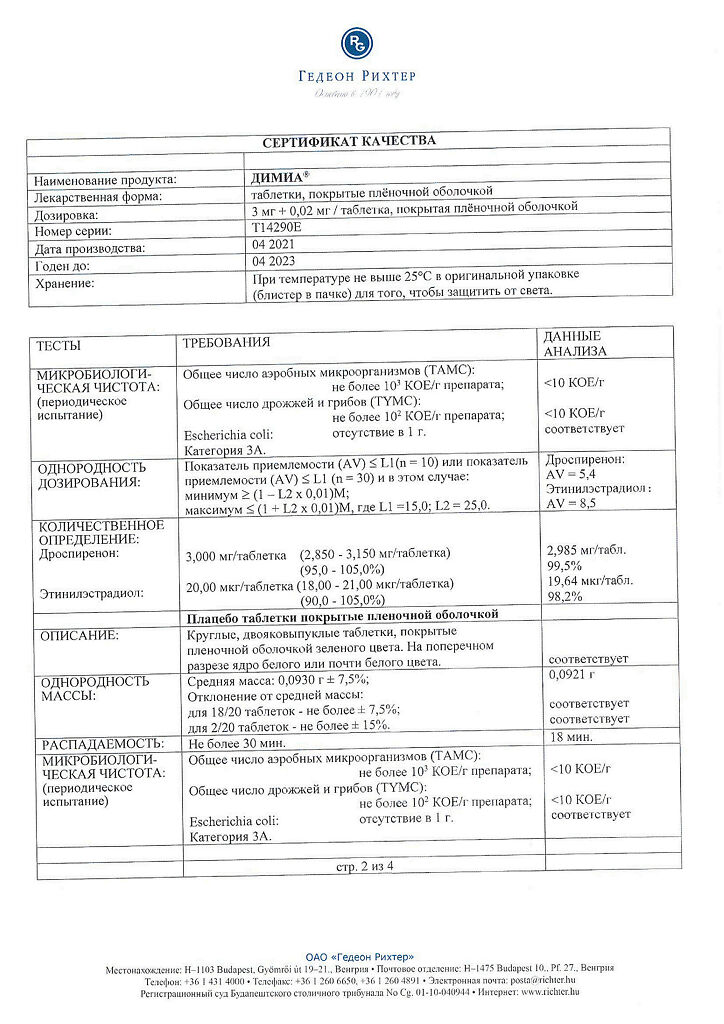
Placebo tablets
1 tablet contains:
MCC – 42.39 mg;
lactose – 37.26 mg;
pregelatinized corn starch – 9 mg;
magnesium stearate – 0.9 mg;
colloidal silicon dioxide – 0.45 mg,
film shell:
Opadry II green 85F21389 (polyvinyl alcohol – 1.2 mg, titanium dioxide – 0.7086 mg, macrogol 3350 – 0.606 mg, talc – 0.444 mg, indigo carmine – 0.0177 mg, quinoline yellow dye – 0.0177 mg, iron oxide dye black – 0.003 mg, dye “Sunset” yellow – 0.003 mg) – 3 mg.
Contraindications
Contraindications
Dimia, like other COCs, is contraindicated in any of the following conditions:
hypersensitivity to the drug or any of the components of the drug;
thrombosis (arterial and venous) and thromboembolism currently or in history (including thrombosis, deep vein thrombophlebitis, pulmonary embolism, myocardial infarction, stroke, cerebrovascular disorders). Conditions preceding thrombosis (including transient ischemic attacks, angina pectoris), currently or in history;
multiple or severe risk factors for venous or arterial thrombosis, incl. complicated lesions of the valvular apparatus of the heart, atrial fibrillation, diseases of the cerebral vessels or coronary arteries; uncontrolled arterial hypertension, major surgery with prolonged immobilization, smoking over the age of 35 years, obesity with a body mass index >30;
hereditary or acquired predisposition to venous or arterial thrombosis, for example, resistance to activated protein C, antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia and antibodies against phospholipids (presence of antibodies to phospholipids – antibodies to cardiolipin, lupus anticoagulant);
pregnancy and suspicion of it;
lactation period;
pancreatitis with severe hypertriglyceridemia currently or in history;
existing (or history of) severe liver disease, provided that liver function is not currently normalized;
severe chronic or acute renal failure;
liver tumor (benign or malignant) currently or in history;
hormone-dependent malignant neoplasms of the genital organs or breast, currently or in history;
bleeding from the vagina of unknown origin;
history of migraine with focal neurological symptoms;
lactase deficiency, lactose intolerance, glucose-galactose malabsorption, Lapp lactase deficiency.
With caution: risk factors for the development of thrombosis and thromboembolism – smoking under the age of 35, obesity, dyslipoproteinemia, controlled arterial hypertension, migraine without focal neurological symptoms, uncomplicated heart valve defects, hereditary predisposition to thrombosis (thrombosis, myocardial infarction or cerebrovascular accident at a young age in one of the immediate relatives); diseases in which peripheral circulatory disorders may occur (diabetes mellitus without vascular complications, systemic lupus erythematosus (SLE), hemolytic-uremic syndrome, Crohn’s disease, ulcerative colitis, sickle cell anemia, phlebitis of the superficial veins); hereditary angioedema; hypertriglyceridemia; severe liver disease (until normalization of liver function tests); diseases that first appeared or worsened during pregnancy or against the background of previous use of sex hormones (including jaundice and/or itching associated with cholestasis, cholelithiasis, otosclerosis with hearing impairment, porphyria, history of herpes during pregnancy, chorea minor (Sydenham disease); chloasma; postpartum period.
Side Effects
Side Effects
Frequency: often (more than 1/100, less than 1/10); uncommon (more than 1/1000, less than 1/100); rare (more than 1/10000, less than 1/1000).
Interaction
Interaction
Note: Before taking concomitant medications, you should read the drug instructions for use to identify potential interactions.
The influence of other drugs on the drug Dimia®. Interactions between oral contraceptives and other drugs may result in acyclic bleeding and/or contraceptive failure. The interactions described below are reflected in the scientific literature.
Mechanism of interaction with hydantoin, barbiturates, primidone, carbamazepine and rifampicin; oxcarbazepine, topiramate, felbamate, ritonavir, griseofulvin and St. John’s wort (Hypericum perforatum) preparations is based on the ability of these active substances to induce microsomal liver enzymes. Maximum induction of liver microsomal enzymes is not achieved within 2–3 weeks, but then persists for at least 4 weeks after cessation of drug therapy.
Contraceptive failure has also been reported with antibiotics such as ampicillin and tetracycline. The mechanism of this phenomenon is unclear. Women during short-term treatment (up to one week) with any of the above groups of drugs or single drugs should temporarily use (during the period of simultaneous use of other drugs and for another 7 days after its end), in addition to COCs, barrier methods of contraception.
Women receiving rifampicin therapy other than COCs should use a barrier method of contraception and continue to use it for 28 days after stopping rifampicin treatment. If taking concomitant medications lasts longer than the expiration date of the active tablets in the package, the inactive tablets should be stopped and immediately started taking drospirenone + ethinyl estradiol tablets from the next package.
If a woman is constantly taking drugs that induce liver microsomal enzymes, she should use other reliable non-hormonal methods of contraception.
The main metabolites of drospirenone in human plasma are formed without the participation of the cytochrome P450 system. Cytochrome P450 inhibitors are therefore unlikely to affect the metabolism of drospirenone.
Effect of Dimia® on other drugs. Oral contraceptives may affect the metabolism of some other active ingredients. Accordingly, the concentrations of these substances in blood plasma or tissues can either increase (for example, cyclosporine) or decrease (for example, lamotrigine). Based on in vitro inhibition studies and in vivo interaction studies in female volunteers taking omeprazole, simvastatin and midazolam as substrates, the effect of drospirenone 3 mg on the metabolism of other active substances is unlikely.
Other interactions. In patients without renal failure, concomitant use of drospirenone and ACE inhibitors or NSAIDs does not have a significant effect on serum potassium levels. However, the simultaneous use of Dimia® with aldosterone antagonists or potassium-sparing diuretics has not been studied. In this case, during the first cycle of treatment, the concentration of serum potassium should be monitored.
Laboratory tests. Taking contraceptive steroids may affect the results of some laboratory tests, including biochemical parameters of liver, thyroid, adrenal and renal function, concentrations of plasma proteins (transporters), such as corticosteroid binding proteins and lipid/lipoprotein fractions, parameters of carbohydrate metabolism, and parameters of blood coagulation and fibrinolysis. In general, changes remain within the normal range. Drospirenone causes an increase in renin activity in the blood plasma and, due to its slight antimineralocorticoid activity, reduces the concentration of aldosterone in the plasma.
Overdose
Overdose
Cases of overdose of Dimia® have not yet been described.
Based on general experience with COCs, potential symptoms of overdose may include: nausea, vomiting, and mild vaginal bleeding.
Treatment: there are no antidotes. Further treatment should be symptomatic.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 25°C.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Gedeon Richter, Hungary
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, protected from light at a temperature not exceeding 25 ° C. |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | pills |
| Brand | Gedeon Richter |
Other forms…
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Buy Dimia, 3 mg+0.02 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.