No products in the cart.
Digoxin, tablets 250 mcg 30 pcs
€1.52 €1.38
Description
A cardiac glycoside. It has a positive inotropic effect. This is due to a direct inhibitory effect on Na+/K+-ATPase on the membrane of cardiomyocytes, which leads to an increase in intracellular sodium ions and, consequently, a decrease in potassium ions. The increased content of sodium ions causes activation of sodium-calcium metabolism, increases the content of calcium ions, resulting in increased strength of myocardial contraction.
As a result of increased contractility of the myocardium, the stroke volume of the blood increases. The final systolic and final diastolic volumes of the heart decrease, which, along with an increase in myocardial tone, leads to a reduction in myocardial size and thus decreases myocardial oxygen demand. It has a negative chronotropic effect, reduces excessive sympathetic activity by increasing the sensitivity of the cardiopulmonary baroreceptors.
With increased activity of the vagus nerve, it has antiarrhythmic effect due to the decrease of the pulse rate through the atriventricular node and the prolongation of the effective refractory period. This effect is enhanced by direct action on the atrioventricular node and sympatholytic action.
The negative dromotropic effect is manifested by an increase in refractoriness of the atrioventricular node, which allows use in paroxysms of supraventricular tachycardia and tachyarrhythmias.
In atrial fibrillation tachyarrhythmia helps to slow the rate of ventricular contractions, prolongs diastole, improves intracardiac and systemic hemodynamics.
Positive butotropic effects are evident in the administration of subtoxic and toxic doses.
It has a direct vasoconstrictor effect that is most clearly seen in the absence of congestive peripheral edema.
At the same time, the indirect vasodilatory effect (in response to increased minute blood volume and reduced excessive sympathetic stimulation of vascular tone) generally prevails over the direct vasoconstrictor effect, resulting in a decrease in total peripheral vascular resistance (TPR).
Indications
Indications
as part of complex therapy of chronic heart failure II (in the presence of clinical manifestations) and III-IV functional class;
tachysystolic form of atrial fibrillation and flutter of paroxysmal and chronic course (especially in combination with chronic heart failure).
Pharmacological effect
Pharmacological effect
Cardiac glycoside. Has a positive inotropic effect. This is due to a direct inhibitory effect on Na+/K+-ATPases on the cardiomyocyte membrane, which leads to an increase in the intracellular content of sodium ions and, accordingly, a decrease in potassium ions. The increased content of sodium ions causes activation of sodium-calcium metabolism, an increase in the content of calcium ions, as a result of which the force of myocardial contraction increases.
As a result of an increase in myocardial contractility, the stroke volume of blood increases. The end-systolic and end-diastolic volumes of the heart decrease, which, along with an increase in myocardial tone, leads to a reduction in its size and thus to a decrease in myocardial oxygen demand. It has a negative chronotropic effect, reduces excessive sympathetic activity by increasing the sensitivity of cardiopulmonary baroreceptors.
Due to the increase in the activity of the vagus nerve, it has an antiarrhythmic effect due to a decrease in the speed of impulses through the atrioventricular node and a lengthening of the effective refractory period. This effect is enhanced by a direct effect on the atrioventricular node and a sympatholytic effect.
The negative dromotropic effect manifests itself in an increase in the refractoriness of the atrioventricular node, which makes it possible to use it for paroxysms of supraventricular tachycardias and tachyarrhythmias.
In case of atrial fibrillation, it helps to slow down the frequency of ventricular contractions, lengthens diastole, and improves intracardiac and systemic hemodynamics.
A positive bathmotropic effect occurs when subtoxic and toxic doses are prescribed.
It has a direct vasoconstrictor effect, which is most clearly manifested in the absence of congestive peripheral edema.
At the same time, the indirect vasodilating effect (in response to an increase in minute blood volume and a decrease in excessive sympathetic stimulation of vascular tone), as a rule, prevails over the direct vasoconstrictor effect, resulting in a decrease in total peripheral vascular resistance (TPVR).
Special instructions
Special instructions
To avoid side effects resulting from overdose, the patient should be monitored throughout the entire period of treatment with Digoxin. Patients receiving digitalis preparations should not be prescribed calcium preparations for parenteral administration.
The dose of Digoxin should be reduced in patients with chronic cor pulmonale, coronary insufficiency, fluid and electrolyte imbalance, renal or liver failure. Elderly patients also require careful dose selection, especially if they have one or more of the above conditions. It should be taken into account that in these patients, even with impaired renal function, creatinine clearance (CC) values may be within normal limits, which is associated with a decrease in muscle mass and a decrease in creatinine synthesis. Since pharmacokinetic processes are disrupted in renal failure, dose selection should be carried out under the control of the concentration of Digoxin in the blood serum. If this is not feasible, then you can use the following recommendations. The dose should be reduced by approximately the same percentage as the QC is reduced. If QC is not determined, then it can be approximately calculated based on the serum creatinine concentration (CCC). For men according to the formula (140 – age)/KKS. For women, the result should be multiplied by 0.85.
In severe renal failure, serum Digoxin concentrations should be determined every 2 weeks, at least during the initial period of treatment.
In case of idiopathic subaortic stenosis (obstruction of the outflow tract of the left ventricle by an asymmetrically hypertrophied interventricular septum), the administration of Digoxin leads to an increase in the severity of obstruction. With severe mitral stenosis and normo- or bradycardia, heart failure develops due to a decrease in diastolic filling of the left ventricle. Digoxin, increasing the contractility of the right ventricular myocardium, causes a further increase in pressure in the pulmonary artery system, which can provoke pulmonary edema and aggravate left ventricular failure. For patients with mitral stenosis, cardiac glycosides are prescribed when right ventricular failure occurs or in the presence of atrial fibrillation.
In patients with second degree AV block, the administration of cardiac glycosides can aggravate it and lead to the development of a Morgagni-Adams-Stokes attack. The prescription of cardiac glycosides for first-degree AV block requires caution, frequent ECG monitoring, and in some cases, pharmacological prophylaxis with agents that improve AV conduction.
Digoxin in Wolff-Parkinson-White syndrome, by slowing down AV conduction, promotes the conduction of impulses through accessory pathways, bypassing the AV node and, thereby, provokes the development of paroxysmal tachycardia. The likelihood of glycoside intoxication increases with hypokalemia, hypomagnesemia, hypercalcemia, hypernatremia, hypothyroidism, severe dilatation of the heart cavities, “pulmonary” heart, myocarditis and in the elderly.
As one of the methods for monitoring the digitalization content when prescribing cardiac glycosides, monitoring their plasma concentration is used.
Cross Sensitivity
Allergic reactions to digoxin and other digitalis drugs are rare. If hypersensitivity to any one digitalis drug occurs, other representatives of this group can be used, since cross-sensitivity to digitalis drugs is not typical.
The patient must strictly follow the following instructions:
1. Use the drug only as prescribed by your doctor, do not change the dose yourself;
2. Use the drug every day only at the appointed time;
3. If your heart rate is below 60 beats/min, you should consult your doctor immediately;
4. If the next dose of the drug is missed, it must be taken immediately, when possible;
5. Do not increase or double the dose;
6. If the patient has not taken the drug for more than 2 days, the doctor must be informed about this.
Before stopping use of the drug, you must inform your doctor.
If vomiting, nausea, diarrhea, or rapid pulse occur, you should immediately consult a doctor.
Before surgery or emergency care, you should be warned about the use of Digoxin.
It is not advisable to use other medications without a doctor’s permission.
Active ingredient
Active ingredient
Digoxin
Composition
Composition
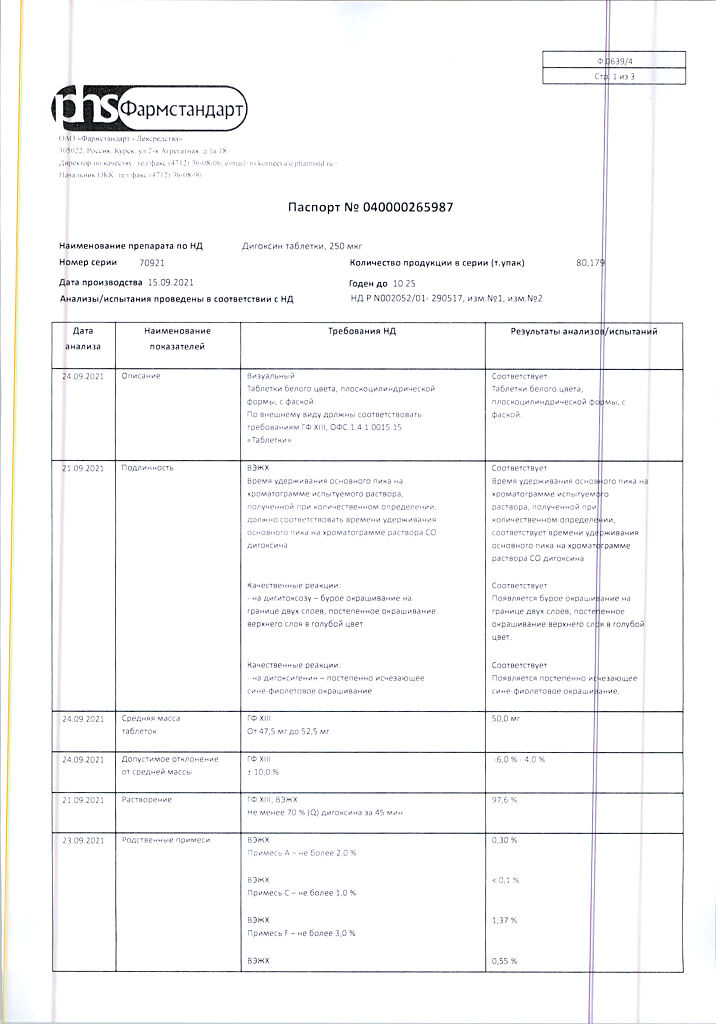
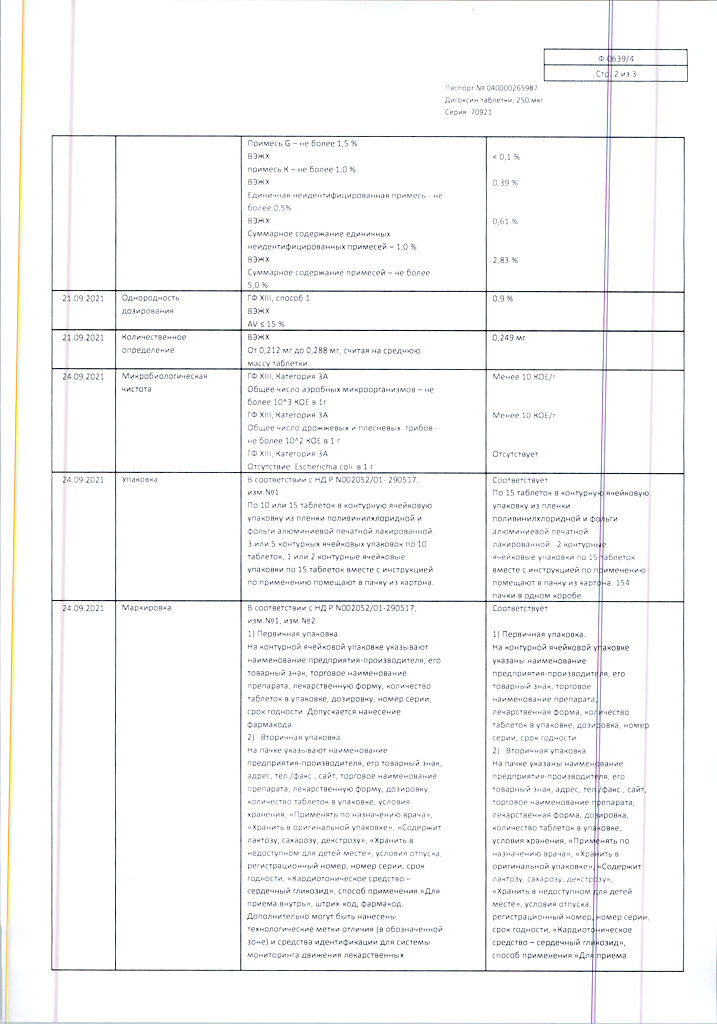
1 tab. digoxin 250 mcg
Contraindications
Contraindications
glycoside intoxication;
Wolff-Parkinson-White syndrome;
2nd degree AV block;
intermittent complete blockade;
hypersensitivity to the drug.
With caution (it is necessary to compare the expected benefits and potential risks): AV block of the first degree, sick sinus syndrome without a pacemaker, the possibility of unstable conduction through the AV node, a history of Morgagni-Adams-Stokes attacks; hypertrophic subaortic stenosis, isolated mitral stenosis with a rare heart rate, cardiac asthma in patients with mitral stenosis (in the absence of the tachysystolic form of atrial fibrillation), acute myocardial infarction, unstable angina, arteriovenous shunt, hypoxia, heart failure with impaired diastolic function (restrictive cardiomyopathy, amyloidosis of the heart, constrictive pericarditis, cardiac tamponade), extrasystole, severe dilatation of the heart cavities, pulmonary heart disease.
Electrolyte disturbances: hypokalemia, hypomagnesemia, hypercalcemia, hypernatremia. Hypothyroidism, alkalosis, myocarditis, old age, renal and hepatic failure, obesity.
Side Effects
Side Effects
The observed side effects are often the initial signs of an overdose.
Symptoms of digitalis intoxication
From the cardiovascular system: ventricular paroxysmal tachycardia, ventricular extrasystole (often bigeminy, polytopic ventricular extrasystole), nodal tachycardia, sinus bradycardia, sinoauricular block, atrial fibrillation and flutter, AV block, on the ECG – a decrease in the ST segment with the formation of a biphasic T wave.
From the digestive system: anorexia, nausea, vomiting, diarrhea, abdominal pain, intestinal necrosis.
From the central nervous system: sleep disturbances, headache, dizziness, neuritis, radiculitis, manic-depressive syndrome, paresthesia and fainting, rarely (mainly in elderly patients suffering from atherosclerosis) – disorientation, confusion, single-color visual hallucinations.
From the side of the organ of vision: coloring of visible objects in a yellow-green color, flickering of “floaters” before the eyes, decreased visual acuity, macro- and micropsia.
Allergic reactions: possible skin rash, rarely – urticaria.
From the hematopoietic system and hemostasis: thrombocytopenic purpura, nosebleeds, petechiae.
Other: hypokalemia, gynecomastia.
Interaction
Interaction
When Digoxin is co-administered with drugs that cause electrolyte imbalance, in particular hypokalemia (for example, diuretics, glucocorticosteroids, insulin, beta-adrenergic agonists, amphotericin B), the risk of arrhythmias and the development of other toxic effects of Digoxin increases. Hypercalcemia can also lead to the development of toxic effects of Digoxin, so intravenous administration of calcium salts should be avoided in patients taking digoxin. In these cases, the dose of Digoxin must be reduced. Certain drugs may increase serum digoxin concentrations, such as quinidine, calcium channel blockers (especially verapamil), amiodarone, spironolactone, and triamterene.
Absorption of Digoxin in the intestine can be reduced by the action of cholestyramine, colestipol, aluminum-containing antacids, neomycin, and tetracyclines. There is evidence that the simultaneous use of spironolactone not only changes the concentration of Digoxin in the blood serum, but can also distort the results of determining the concentration of Digoxin, therefore special care is required when assessing the results obtained.
A decrease in the bioavailability of Digoxin is observed when administered simultaneously with activated carbon, astringents, kaolin, sulfasalazine (binding in the gastrointestinal lumen), metoclopramide, proserin (increased gastrointestinal motility).
An increase in the bioavailability of Digoxin is observed when administered simultaneously with broad-spectrum antibiotics that suppress intestinal microflora (reducing destruction in the gastrointestinal tract).
Beta-blockers and verapamil increase the severity of the negative chronotropic effect and reduce the strength of the inotropic effect.
Inducers of microsomal oxidation (barbiturates, phenylbutazone, phenytoin, rifampicin, antiepileptics, oral contraceptives) can stimulate the metabolism of Digoxin (if they are discontinued, digitalis intoxication is possible).
When used simultaneously with digoxin, the following drugs may interact, as a result of which the therapeutic effect is reduced or the side or toxic effect of Digoxin appears: mineralocorticoids, glucocorticoids with a significant mineralocorticoid effect, amphotericin B for injection, carbonic anhydrase inhibitors, adrenocorticotropic hormone (ACTH), diuretics that promote the release of water and potassium (bumetadine, ethacrynic acid, furosemide, indapamide, mannitol and thiazide derivatives), sodium phosphate.
Hypokalemia caused by these drugs increases the risk of toxic effects of Digoxin, therefore, when used simultaneously with Digoxin, constant monitoring of potassium concentration in the blood is required.
When administered simultaneously with St. John’s wort, P-glycoprotein and cytochrome P450 are induced and, consequently, bioavailability decreases, metabolism increases and the concentration of digoxin in plasma decreases markedly.
When administered simultaneously with amiodarone, the concentration of digoxin in the blood plasma increases to a toxic level. The interaction of amiodarone and digoxin inhibits the activity of the sinus and atrioventricular nodes of the heart, and also slows down the conduction of nerve impulses through the conduction system of the heart. Therefore, when prescribing amiodarone, it is necessary to discontinue Digoxin or reduce the dose by half.
Preparations of aluminum and magnesium salts and other antacids can reduce the absorption of digoxin and reduce its concentration in the blood.
The simultaneous use of antiarrhythmic drugs, calcium salts, pancuronium, rauwolfia alkaloids, succinylcholine and sympathomimetics with digoxin can provoke the development of cardiac arrhythmias, therefore in these cases it is necessary to monitor the patient’s cardiac activity and ECG.
Kaolin, pectin and other adsorbents, cholestyramine, colestipol, laxatives, neomycin and sulfasalazine reduce the absorption of Digoxin and thereby reduce its therapeutic effect.
Blockers of slow calcium channels, captopril – increase the concentration of Digoxin in the blood plasma, therefore, when used together, it is necessary to reduce the dose of Digoxin to avoid the toxic effect of the latter.
Edrophonium (an anticholinesterase drug) increases the tone of the parasympathetic nervous system, so its interaction with digoxin can cause severe bradycardia.
Erythromycin improves the absorption of Digoxin in the intestine.
Digoxin reduces the anticoagulant effect of heparin, so the dose of heparin should be increased when administered simultaneously with Digoxin.
Indomethacin reduces the release of Digoxin, so the danger of toxic effects of the latter increases.
Magnesium sulfate solution for injection is used to reduce the toxic effects of cardiac glycosides.
Phenylbutazone reduces the concentration of digoxin in the blood serum.
Potassium salt preparations should not be taken if conduction disturbances on the ECG appear under the influence of Digoxin. However, potassium salts are often prescribed along with digitalis preparations to prevent heart rhythm disturbances.
Quinidine and quinine can sharply increase Digoxin concentrations.
Spironolactone reduces the rate of elimination of Digoxin, so when used together, the dose of Digoxin should be adjusted.
When studying myocardial perfusion with thalium drugs (Thalium chloride) in patients taking Digoxin, the degree of thalium accumulation in areas of damage to the heart muscle is reduced and the study results are distorted.
Thyroid hormones increase metabolism, so the dose of Digoxin should be increased.
Overdose
Overdose
Symptoms: decreased appetite, nausea, vomiting, diarrhea, abdominal pain, intestinal necrosis, ventricular paroxysmal tachycardia, ventricular extrasystole (often polytopic or bigeminy), nodal tachycardia, sinoatrial block, atrial fibrillation and flutter, AV block, drowsiness, confusion, delirious psychosis, decreased visual acuity, coloring of visible objects in a yellow-green color, flashing “flies” before the eyes, perception of objects in a reduced or enlarged form, neuritis, radiculitis, manic-depressive syndrome, paresthesia.
Treatment: withdrawal of digoxin, administration of activated charcoal (to reduce absorption), administration of antidotes (unithiol, EDTA, antibodies to digoxin), symptomatic therapy. Continuous ECG monitoring is carried out.
In cases of hypokalemia, potassium salts are widely used: 0.5-1 g of potassium chloride is dissolved in water and taken several times a day for a total dose of 3-6 g (40-80 mEq of potassium) for adults provided that renal function is adequate. In emergency cases, intravenous drip administration of 2% or 4% potassium chloride solution is indicated. The daily dose is 40-80 mEq of potassium (diluted to a concentration of 40 mEq of potassium per 500 ml). The recommended rate of administration should not exceed 20 mEq/h (under ECG monitoring). For hypomagnesemia, the administration of magnesium salts is recommended.
In cases of ventricular tachyarrhythmia, slow intravenous administration of lidocaine is indicated. In patients with normal cardiac and renal function, slow intravenous administration (over 2-4 minutes) of lidocaine at an initial dose of 1-2 mg/kg body weight, followed by drip administration at a rate of 1-2 mg/min, is usually effective. In patients with impaired renal and/or cardiac function, the dose should be reduced accordingly.
In the presence of II-III degree AV blockade, lidocaine and potassium salts should not be prescribed until an artificial pacemaker is installed.
During treatment, it is necessary to monitor the level of calcium and phosphorus in the blood and daily urine.
There is experience with the use of the following drugs with possible positive effects: β-blockers, procainamide, bretylium and phenytoin. Cardioversion may precipitate ventricular fibrillation. The use of atropine is indicated for the treatment of bradyarrhythmias and AV block. In cases of II-III degree AV block, asystole and suppression of sinus node activity, installation of a pacemaker is indicated.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 30°C.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 30 ° C. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | pills |
| Brand | Pharmstandard-Leksredstva |
Related products
Buy Digoxin, tablets 250 mcg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.