No products in the cart.
Diclofenac retard Obolenskoye, 100 mg 20 pcs
€3.25 €2.04
Description
Pharmgroup:
NSAIDs.
Pharmic action:
Diclofenac retard is a nonsteroidal anti-inflammatory drug (NSAID), a phenylacetic acid derivative; it has anti-inflammatory, analgesic and antipyretic effects.
Inhibiting cyclooxygenase 1 and 2 (COX1 and COX2) indiscriminately, it disrupts arachidonic acid metabolism, reduces the amount of prostaglandins in the inflammation focus. It is most effective for pain of inflammatory nature. Like all NSAIDs, the drug has anti-aggregant effect. In rheumatic diseases, anti-inflammatory and analgesic effect of diclofenac contributes to a significant reduction in the severity of pain, morning stiffness, joint swelling, which improves the functional state of the joint. In case of injuries, in post-operative period diclofenac reduces pain and inflammatory edema.
Pharmacokinetics:
Absorption is rapid and complete. Food slows the rate of absorption by 1-4 h and reduces the maximum concentration (Cmax) by 40%.
As a result of delayed release of the drug, Cmax in plasma is lower than that produced by short-acting drugs (drugs); however, it remains high for a long time after administration. Cmax is 0.5-1.0 mcg/ml, time to reach maximum plasma concentration (TCmax) is 5 h after administration of 100 mg long-acting tablets.
There is no change in pharmacokinetics of diclofenac on repeated administration. It does not cumulate if the recommended interval between meals is observed.
The bioavailability is 50%. Binding with plasma proteins is more than 99% (most of it is bound with albumin). It penetrates into breast milk and synovial fluid; Cmax in synovial fluid is observed 2-4 hours later than in plasma. Period of half-life (T1/2) from synovial fluid – 3-6 h (concentration of the drug in synovial fluid 4-6 h after administration is higher than in plasma and stays higher during 12 h).
50% of the active substance is metabolized during the “first passage” through the liver. Metabolism occurs as a result of multiple or single hydroxylation and conjugation with glucuronic acid. The enzyme system of cytochrome P 450 CYP2C9 is involved in metabolism of the drug. The pharmacological activity of the metabolites is less than that of diclofenac.
The systemic clearance is 206 ml/min. T1/2 from plasma is 1-2 hours. 60% of the administered dose is excreted as metabolites through the kidneys; less than 1% is excreted unchanged, the rest of the dose is excreted as metabolites in the bile.
In patients with significant renal insufficiency (creatinine clearance (CK) less than 10 ml/min) excretion of metabolites in bile is increased, while there is no increase of their concentrations in blood.
In patients with chronic hepatitis or compensated liver cirrhosis pharmacokinetic parameters are not changed.
Diclofenac penetrates into the breast milk.
Indications
Indications
Diseases of the musculoskeletal system (rheumatoid arthritis, psoriatic, juvenile chronic arthritis, ankylosing spondylitis (Bechterew’s disease); gouty arthritis; rheumatic soft tissue lesions; osteoarthritis of peripheral joints and spine, including with radicular syndrome; tenosynovitis; bursitis).
Pain syndrome of mild or moderate severity: neuralgia, myalgia, lumboischialgia, post-traumatic pain syndrome accompanied by inflammation, postoperative pain, headache, migraine, algomenorrhea, adnexitis, proctitis, toothache.
As part of complex therapy for infectious and inflammatory diseases of the ear, nose and throat with severe pain (pharyngitis, tonsillitis, otitis media).
The drug Diclofenac retard is intended for symptomatic therapy, reducing pain and inflammation at the time of use, and does not affect the progression of the disease.
Pharmacological effect
Pharmacological effect
Pharmaceutical group:
NSAIDs.
Pharmaceutical action:
Diclofenac retard is a non-steroidal anti-inflammatory drug (NSAID), a derivative of phenylacetic acid; has anti-inflammatory, analgesic and antipyretic effects.
By indiscriminately inhibiting cyclooxygenase 1 and 2 (COX1 and COX2), it disrupts the metabolism of arachidonic acid and reduces the amount of prostaglandins at the site of inflammation. Most effective for inflammatory pain. Like all NSAIDs, the drug has an antiplatelet effect. In rheumatic diseases, the anti-inflammatory and analgesic effect of diclofenac helps to significantly reduce the severity of pain, morning stiffness, and swelling of the joints, which improves the functional state of the joint. For injuries, in the postoperative period, diclofenac reduces pain and inflammatory swelling.
Pharmacokinetics:
Absorption is fast and complete. Food slows the rate of absorption by 1-4 hours and reduces the maximum concentration (Cmax) by 40%.
As a result of the delayed release of the drug, Cmax in plasma is lower than that created by the administration of short-acting drugs; however, it remains high for a long time after administration. Cmax – 0.5-1.0 mcg/ml, time to reach maximum concentration in blood plasma (TCmax) – 5 hours after taking 100 mg extended-release tablets.
There are no changes in the pharmacokinetics of diclofenac following repeated administration. Does not accumulate if the recommended interval between meals is observed.
Bioavailability – 50%. Communication with plasma proteins is more than 99% (most of it is associated with albumin). Penetrates into breast milk and synovial fluid; Cmax in synovial fluid is observed 2-4 hours later than in plasma. The half-life (T1/2) from synovial fluid is 3-6 hours (concentrations of the drug in synovial fluid 4-6 hours after its administration are higher than in plasma and remain higher for another 12 hours).
50% of the active substance is metabolized during the “first pass” through the liver. Metabolism occurs as a result of multiple or single hydroxylation and conjugation with glucuronic acid. The enzyme system of cytochrome P 450 CYP2C9 takes part in the metabolism of the drug. The pharmacological activity of the metabolites is less than that of diclofenac.
Systemic clearance is 206 ml/min. T1/2 from plasma – 1-2 hours. 60% of the administered dose is excreted in the form of metabolites through the kidneys; less than 1% is excreted unchanged, the rest of the dose is excreted as metabolites in the bile.
In patients with severe renal failure (creatinine clearance (CC) less than 10 ml/min), the excretion of metabolites in bile increases, but no increase in their concentration in the blood is observed.
In patients with chronic hepatitis or compensated liver cirrhosis, pharmacokinetic parameters do not change.
Diclofenac passes into breast milk.
Special instructions
Special instructions
Prescribe with extreme caution for severe renal, hepatic and heart failure. Long-term use of Diclofenac sodium can cause disorders of the kidneys, liver and gastrointestinal tract. Patients who experience deterioration in liver function during the use of diclofenac sodium, or who experience increased activity of liver enzymes, should discontinue use of the drug.
Patients should take Diclofenac after meals to avoid irritation in the gastrointestinal tract.
The use of Diclofenac sodium requires caution when driving a car and operating machinery due to its effect on the central nervous system.
Active ingredient
Active ingredient
Diclofenac
Composition
Composition
Active ingredient:
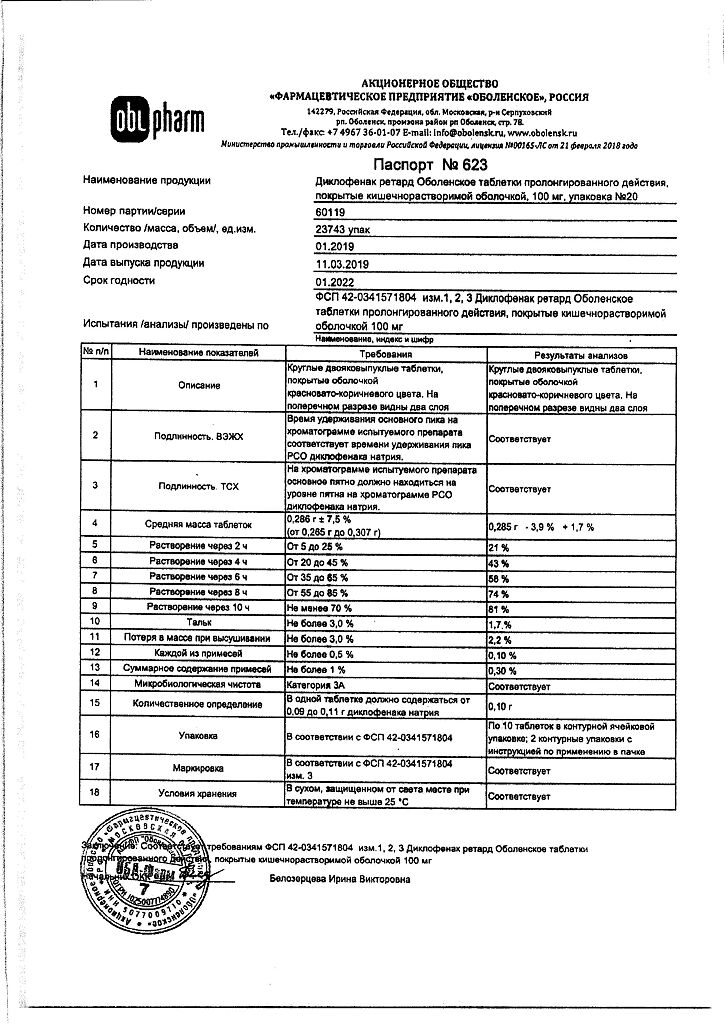
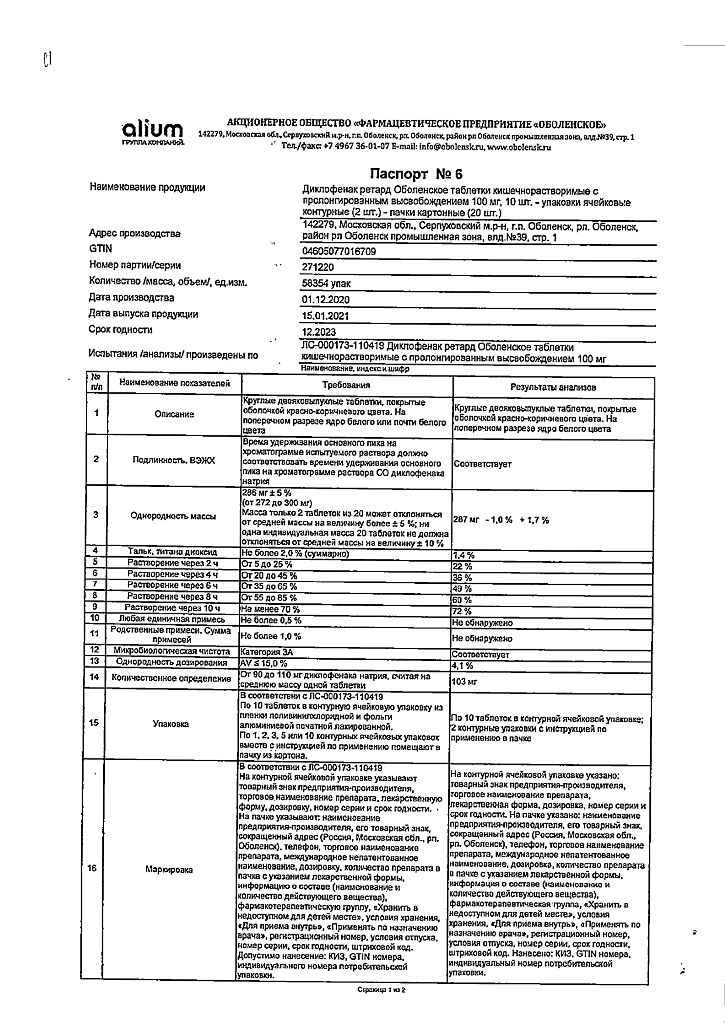
diclofenac sodium 100 mg
Pregnancy
Pregnancy
Diclofenac should not be taken during pregnancy and should only be prescribed in urgent cases. It is excreted in breast milk, so should not be taken during breastfeeding.
Contraindications
Contraindications
Erosive and ulcerative lesions of the gastrointestinal tract in the acute phase, “aspirin triad”, hematopoietic disorders of unknown etiology, hypersensitivity to diclofenac and components of the dosage form used, or other NSAIDs.
Side Effects
Side Effects
From the digestive system: nausea, vomiting, epigastric pain, anorexia, flatulence, constipation, gastritis up to erosive with bleeding, increased transaminase activity, drug-induced hepatitis, pancreatitis.
From the urinary system: interstitial nephritis.
From the side of the central nervous system: headache, dizziness, disorientation, agitation, insomnia, irritability, fatigue, aseptic meningitis.
From the respiratory system: bronchospasm.
From the hematopoietic system: anemia, thrombocytopenia, leukopenia, agranulocytosis.
Dermatological reactions: exanthema, erythema, eczema, hyperemia, erythroderma, photosensitivity.
Allergic reactions: erythema multiforme, Lyell’s syndrome, Stevens-Johnson syndrome, anaphylactic reactions, including shock.
Local reactions: burning, formation of infiltrate, and necrosis of adipose tissue are possible at the injection site.
Other: fluid retention in the body, edema, increased blood pressure.
Interaction
Interaction
With the simultaneous use of Diclofenac with digoxin, phenytoin or lithium preparations, it is possible to increase the plasma concentrations of these drugs; with diuretics and antihypertensive drugs – the effect of these drugs may be reduced; with potassium-sparing diuretics – hyperkalemia may develop; with acetisalicylic acid – a decrease in the concentration of diclofenac in the blood plasma and an increased risk of side effects.
Diclofenac may enhance the toxic effect of cyclosporine on the kidneys.
Diclofenc can cause hypo- or hyperglycemia, therefore, when used simultaneously with hypoglycemic agents, monitoring of blood glucose concentrations is required.
When using methotrexate within 24 hours before or after taking Diclofenac, the concentration of methotrexate may increase and its toxic effect may increase.
When used simultaneously with anticoagulants, regular monitoring of blood clotting parameters is necessary.
Overdose
Overdose
In case of acute Diclofenac poisoning, treatment mainly consists of supportive and symptomatic measures.
Gastric lavage is used to reduce absorption.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature below 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Obolenskoye FP JSC, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature below 25 °C |
| Manufacturer | Obolenskoe FP JSC, Russia |
| Medication form | enteric soluble tablets |
| Brand | Obolenskoe FP JSC |
Related products
Buy Diclofenac retard Obolenskoye, 100 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.