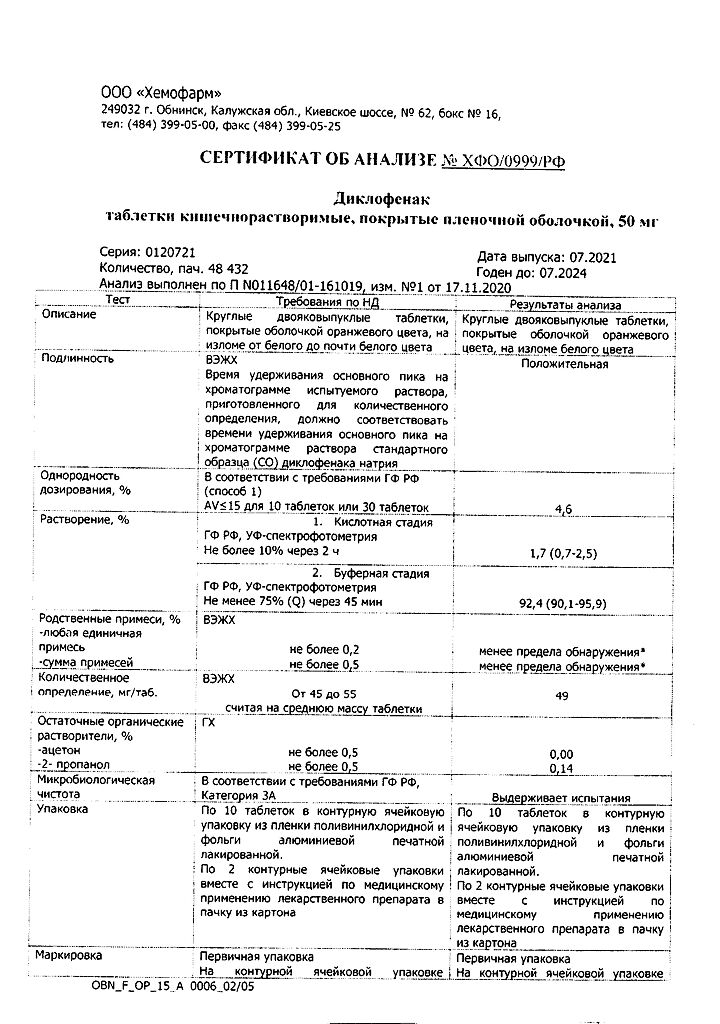
No products in the cart.
Diclofenac, 50 mg 20 pcs
€1.56 €1.42
Description
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID).
It has anti-inflammatory, analgesic and antipyretic effects.
Inhibits COX enzyme in the cascade of arachidonic acid metabolism and disrupts prostaglandin biosynthesis.
When used externally it has anti-inflammatory and analgesic effects.
Limits and relieves pain at the site of application of the ointment (including joint pain at rest and on movement), reduces morning stiffness and joint swelling. Helps to increase the amount of movement.
Absorption is fast and complete, food slows the rate of absorption by 1-4 hours and reduces the maximum concentration (Cmax) by 40%.
After oral administration of 50 mg, the maximum concentration (Cmax) – 1.5 mcg/ml is reached after – 2-3 hours. Plasma concentrations are linearly related to the amount of the administered dose.
There is no change in pharmacokinetics of diclofenac on repeated administration. It does not cumulate if the recommended interval between doses is observed.
The bioavailability is 50%. Binding with plasma proteins is more than 99% (most of it is bound with albumin).
It penetrates into synovial fluid; Cmax in synovial fluid is observed 2-4 hours later than in plasma.
The half-life (T½) of synovial fluid is 3-6 h (the concentration of the active substance in synovial fluid is higher in synovial fluid 4-6 h after administration than in plasma and stays higher for 12 h).
The relationship between the concentration of the drug in synovial fluid and the clinical efficacy of the drug has not been elucidated.
Metabolism: 50% of the active substance is metabolized during the “first passage” through the liver.
Metabolism occurs as a result of multiple or single hydroxylation and conjugation with glucuronic acid.
The CYP2C9 isoenzyme is involved in metabolism of the drug. The pharmacological activity of the metabolites is lower than that of diclofenac.
The systemic clearance is about 260±50 ml/min, the volume of distribution is 550 ml/kg.
T½ from plasma averages about 2.5 hours. 65% of the administered dose is excreted as metabolites by the kidneys; less than 1% is excreted unchanged, the rest of the dose is excreted as metabolites in the bile.
In patients with severe renal insufficiency (creatinine clearance (CK) less than 10 ml/min) excretion of metabolites in bile is increased, while there is no increase in their blood concentrations.
In patients with chronic hepatitis or compensated liver cirrhosis pharmacokinetic parameters of diclofenac are not changed.
Diclofenac penetrates into breast milk.
Indications
Indications
Symptomatic treatment of diseases of the musculoskeletal system (rheumatoid arthritis, psoriatic arthritis, juvenile chronic arthritis, ankylosing spondylitis (Behterev’s disease); gouty arthritis,
rheumatic soft tissue lesions, osteoarthritis of peripheral joints and spine, including radicular syndrome, tendovaginitis, bursitis).
The drug relieves or reduces pain and inflammation during treatment, while not affecting the progression of the disease.
Pain syndrome of mild to moderate severity: neuralgia, myalgia, lumboinsychialgia, post-traumatic pain syndrome accompanied by inflammation, postoperative pain, headache, migraine, algodysmenorrhea, adnexitis, proctitis, toothache.
In the complex treatment of infectious and inflammatory diseases of the ear, throat, nose, with a pronounced pain syndrome (pharyngitis, tonsillitis, otitis).
Active ingredient
Active ingredient
Composition
Composition
One enteric-coated tablet contains:
The active ingredient:
diclofenac sodium 50.0000 mg;
Associates:
Lactose monohydrate – 46.6000 mg,
Corn starch – 59.2000 mg,
Povidone-K30 – 10,0000 mg,
Sodium lauryl sulfate – 10,0000 mg,
/p>
Sodium carboxymethyl starch – 20.0000 mg,
Silicon dioxide colloid – 3.0000 mg,
Magnesium starch – 10.0000 mg./p>
Magnesium stearate – 1,2000 mg;
Shell:
Methacrylic acid and ethyl acrylate copolymer – 4.1379 mg,
Macrogol 6000 – 1.0345 mg,
Talc – 6.7241 mg,
Titanium dioxide E 171 CI 77891 – 1.0345 mg,
Sunset yellow dye [E110] – 2.0670 mg.
How to take, the dosage
How to take, the dosage
In all patients receiving diclofenac, it should be used in the lowest effective dose for the shortest time required to reduce the severity of symptoms.
Overly, without chewing, with or after meals, with plenty of water.
Adults and adolescents from 15 years of age – 50 mg 2-3 times a day.
When optimal therapeutic effect is achieved, the dose is gradually reduced and maintenance treatment is switched to a dose of 50 mg/day. The maximum daily dose is 150 mg.
Interaction
Interaction
Lithium and digoxin drugs: diclofenac may increase plasma concentrations of lithium and digoxin. Monitoring of plasma concentrations of lithium and digoxin in concomitant use with diclofenac is recommended.
Methotrexate: caution is required if diclofenac is administered less than 24 hours before or 24 hours after methotrexate administration as this may increase methotrexate concentration in blood and increase its toxic effect.
Cyclosporine: The effect of diclofenac on prostaglandin synthesis in the kidneys may increase nephrotoxicity of cyclosporine. Therefore, administered doses of diclofenac should be lower than in patients not using cyclosporine.
Diuretic and hypotensive drugs: diclofenac may decrease the hypotensive effect of diuretic and hypotensive drugs (e.g., beta-adrenoblockers, angiotensin converting enzyme inhibitors – ACE). In patients, especially elderly patients, these combinations should be prescribed with caution and blood pressure should be monitored regularly. Patients should be adequately hydrated. After initiation and periodically during treatment, especially when diuretics and ACE inhibitors are prescribed concomitantly, renal function should be monitored because of the increased risk of nephrotoxicity.
Drugs known to cause hyperkalemia: Concomitant use of diclofenac with potassium-saving diuretics, cyclosporine, tacrolimus, or trimethoprim may increase serum potassium concentrations (this should be monitored regularly if such combinations of drugs are used).
Quinolone-derived antibacterials: there have been some reports of seizures in patients receiving quinolone derivatives and diclofenac concomitantly.
Anticoagulants and antiaggregants: caution should be exercised when combining diclofenac with the drugs of these groups because of the risk of bleeding. Although in clinical trials the effect of diclofenac on anticoagulant activity has not been established, there are separate reports about the increase of bleeding risk in patients who have taken this combination of drugs. Therefore, regular and close monitoring of patients is recommended in case of this combination of drugs.
Acetylsalicylic acid reduces the blood concentration of diclofenac.
NSAIDs and corticosteroids: Simultaneous systemic use of diclofenac and other systemic NSAIDs or corticosteroids may increase the incidence of side effects (particularly gastrointestinal side effects).
Selective serotonin reuptake inhibitors (SSRIs): Simultaneous use of diclofenac and drugs from the SSRI group increases the risk of gastrointestinal bleeding.
Hypoglycemic drugs: In clinical trials it has been found that when used together diclofenac does not affect the effectiveness of hypoglycemic drugs. However, some reports are known about the development in such cases of both hypoglycemia and hyperglycemia, which required changing the dose of hypoglycemic drugs during diclofenac therapy. Therefore, it is recommended to monitor blood glucose concentrations during concomitant use of diclofenac and hypoglycemic drugs.
Phenytoin: concomitant use of phenytoin and diclofenac requires monitoring of phenytoin plasma concentrations due to possible intensification of its systemic effects.
Tacrolimus: possible increased nephrotoxicity with diclofenac.
Cefamandole, cefoperazone, cefotetan, valproic acid and plikamycin increase the incidence of hypoprothrombinemia.
The effect of diclofenac on the synthesis of prostaglandins in the kidneys may increase the toxic effects of gold drugs. Concomitant use with ethanol, colchicine, corticotropin and preparations of St. John’s wort increases the risk of gastrointestinal bleeding.
Powerful CYP2C9 isoenzyme inhibitors: caution should be exercised when diclofenac and powerful CYP2C9 isoenzyme inhibitors (such as voriconazole) are co-administered due to possible increase of diclofenac serum concentration and potent systemic action.
Special Instructions
Special Instructions
In order to reduce the risk of adverse events, the drug should be used in the lowest effective dose for the shortest period necessary to relieve symptoms.
Therapy with NSAIDs, including diclofenac, particularly long-term and high-dose therapy, may be associated with a slightly increased risk of serious cardiovascular thrombotic complications (including myocardial infarction and stroke).
In patients with significant cardiovascular risk factors (e.g., arterial hypertension, hyperlipoproteinemia, diabetes mellitus, and smoking), treatment with diclofenac preparations should be initiated only after careful evaluation and assessment.
Because of the important role of prostaglandins in maintaining renal blood flow, special caution should be exercised when prescribing the drug in patients with cardiac or renal insufficiency, hypertension, elderly patients, patients taking diuretics or other drugs that affect renal/p>
and patients who have decreased circulating blood volume for any reason (e.g., after major surgery).
If diclofenac is prescribed in these cases, it is recommended to monitor renal function as a precaution.
After discontinuation of therapy with the drug, normalization of renal function to baseline values is usually noted.
When using diclofenac, there have been events such as bleeding or gastrointestinal ulcers/perforations, in some cases with fatal outcome.
These events can occur at any time when using the drug in patients with or without pre-existing symptoms and a history of serious gastrointestinal disease.
In elderly patients, such complications may have serious consequences.
If patients receiving diclofenac develop gastrointestinal bleeding or ulceration, the drug should be discontinued.
In order to reduce the risk of gastrointestinal toxicity, the drug should be used in the lowest effective dose for the shortest time possible, particularly in patients with gastrointestinal ulcers, especially those with a history of bleeding or perforation, and in older patients.
Patients at increased risk of gastrointestinal complications, as well as those receiving therapy with low-dose acetylsalicylic acid or other medications that can increase the risk of gastrointestinal damage, should take gastroprotective agents.
Patients with a history of gastrointestinal damage, especially the elderly, should inform their physician of any digestive symptoms.
Long-term therapy should include monitoring of liver function, peripheral blood count, and fecal occult blood tests.
In prolonged use of diclofenac an increase in activity of one or more “liver” enzymes may be noted.
If liver function abnormalities persist and progress or signs of liver disease or other symptoms (e.g., eosinophilia, rash, etc.) the drug must be stopped.
It should be noted that hepatitis with diclofenac may occur without prodromal signs.
Precautions must be taken when using diclofenac in patients with hepatic porphyria because the drug may provoke attacks of porphyria.
Diclofenac may reversibly inhibit platelet aggregation; therefore, in patients with hemostasis disorders during long-term use it is necessary to monitor closely the corresponding laboratory parameters.
In patients with bronchial asthma, seasonal allergic rhinitis, nasal mucosal edema (including with nasal polyps), chronic obstructive pulmonary disease, chronic respiratory tract infections (especially those associated with allergic rhinitis-like symptoms), and
In patients with allergies to other medications (rash, itching, urticaria) special caution must be exercised when diclofenac is prescribed (readiness for resuscitation).
In the use of diclofenac, severe, in some cases fatal, skin reactions, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis have very rarely been reported.
The highest risk and incidence of severe dermatologic reactions were noted in the first month of treatment with diclofenac.
If patients receiving the drug develop the first signs of skin rash, mucosal lesions or other symptoms of hypersensitivity, diclofenac should be discontinued.
The anti-inflammatory effects of NSAIDs, including diclofenac, can make it difficult to diagnose infections.
Due to the negative effect on fertility, it is not recommended for women planning to become pregnant.
In patients with infertility (including those undergoing examination) it is recommended to stop the drug.
Synopsis
Synopsis
Features
Features
Contraindications
Contraindications
With caution:
Anemia, bronchial asthma, confirmed chronic heart failure NYHA functional class I, arterial hypertension, edema syndrome, hepatic or renal failure (creatinine clearance 30-60 ml/min), dyslipidemia, hyperlipoproteinemia, diabetes, smoking,
Inflammatory bowel disease, conditions after major surgical interventions, inducible porphyria, diverticulitis, systemic connective tissue diseases, pregnancy I-II trimester.
Anamnestic evidence of gastrointestinal ulcers, Helicobacter pylori infection, older age, long-term use of NSAIDs, heavy alcohol use, and severe medical conditions.
Concomitant therapy with anticoagulants (e.g., warfarin), antiaggregants (e.g., acetylsalicylic acid, clopidogrel), oral glucocorticosteroids (e.g., prednisolone),
selective serotonin reuptake inhibitors (e.g., citalopram, fluoxetine, paroxetine, sertraline).
In patients with seasonal allergic rhinitis, nasal mucosal edema (including with nasal polyps), chronic obstructive pulmonary disease, chronic respiratory tract infections (especially
associated with allergic rhinitis-like symptoms), with allergy to other medications, in patients with significant reduction of circulating blood volume, diclofenac should be used with caution.
Side effects
Side effects
Side effects depend on individual sensitivity, the amount of dose used and the duration of treatment.
Digestive system disorders:nausea, vomiting, epigastric pain, anorexia, flatulence, constipation, gastritis up to erosive with bleeding, increased transaminase activity, drug hepatitis, pancreatitis.
With the urinary system:interstitial nephritis.
CNS side:headache, dizziness, disorientation, agitation, insomnia, irritability, fatigue, aseptic meningitis.
In the respiratory system: bronchospasm.
Hematopoietic system disorders:anemia, thrombocytopenia, leukopenia, agranulocytosis.
Dermatological reactions: eczema, erythema, eczema, hyperemia, erythroderma, photosensitization.
Allergic reactions: erythema multiforme, Lyell’s syndrome, Stevens-Johnson syndrome, anaphylactic reactions, including shock.
Local reactions:in the injection site, burning, infiltrate formation, necrosis of adipose tissue may occur.
Other: fluid retention in the body, edema, increased BP.
In external use
The place of application of the ointment may cause skin rash, burning, redness.
Long-term external use may cause systemic adverse effects on the digestive system, central nervous system, respiratory system and allergic reactions.
.
Overdose
Overdose
Symptoms: vomiting, gastrointestinal bleeding, diarrhea, dizziness, tinnitus, seizures, increased BP, respiratory depression, with significant overdose – acute renal failure, hepatotoxic effect.
Treatment: gastric lavage, activated charcoal, symptomatic therapy aimed at elimination of BP increase, renal dysfunction, seizures, gastrointestinal damage, respiratory depression. Forced diuresis, hemodialysis are ineffective (due to significant binding to proteins and intense metabolism).
Pregnancy use
Pregnancy use
There are insufficient data on the safety of diclofenac use in pregnant women.
Therefore, diclofenac should be administered in the first and second trimesters of pregnancy only when the expected benefit to the mother exceeds the potential risk to the fetus.
Diclofenac, like other prostaglandin synthesis inhibitors, is contraindicated in the last 3 months of pregnancy (inhibition of uterine contractility and premature closure of the fetal arterial duct may occur).
Diclofenac passes in small amounts into breast milk. To prevent undesirable effects on the baby, the drug should not be prescribed to breastfeeding women.
Breast-feeding should be stopped if the drug has to be used.
Similarities
Similarities
Additional information
| Weight | 0.012 kg |
|---|---|
| Shelf life | 3 years. Do not use after the expiration date stated on the package. |
| Conditions of storage | In a place protected from light and moisture, at 15-25 °C |
| Manufacturer | Chemopharm LLC, Russia |
| Medication form | enteric soluble tablets |
| Brand | Chemopharm LLC |
Other forms…
Related products
Buy Diclofenac, 50 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.