No products in the cart.
Description
Desal is a blocker of histamine H1-receptors (long-acting). It is the primary active metabolite of loratadine. Inhibits release of histamine and leukotriene C4 from mast cells. It prevents the development and facilitates the course of allergic reactions.
It has anti-allergic, antipruritic and antiexudative action. It reduces capillary permeability, prevents the development of tissue edema, relieves spasm of smooth muscles. It has practically no sedative effect and does not affect the speed of psychomotor reactions when administered at a dose of 7.5 mg. In comparative studies of desloratadine and loratadine no qualitative or quantitative differences in toxicity of the two drugs in comparable doses (taking into account the concentration of desloratadine) were found.
Pharmacokinetics
After oral administration begins to be determined in plasma after 30 minutes. Food has no effect on distribution. Bioavailability is proportional to the dose in the range of 5 mg to 20 mg.
The binding to plasma proteins is 83-87%. It does not penetrate the blood-brain barrier (BBB). It is extensively metabolized in liver by hydroxylation with formation of 3-ON-desloratadine combined with glucuronide, only small part of oral dose is excreted by kidneys (less than 2%) and with feces (less than 7%).
Indications
Indications
Symptomatic therapy of seasonal and year-round allergic rhinitis, chronic idiopathic urticaria.
Active ingredient
Active ingredient
Composition
Composition
1 ml of the solution contains:
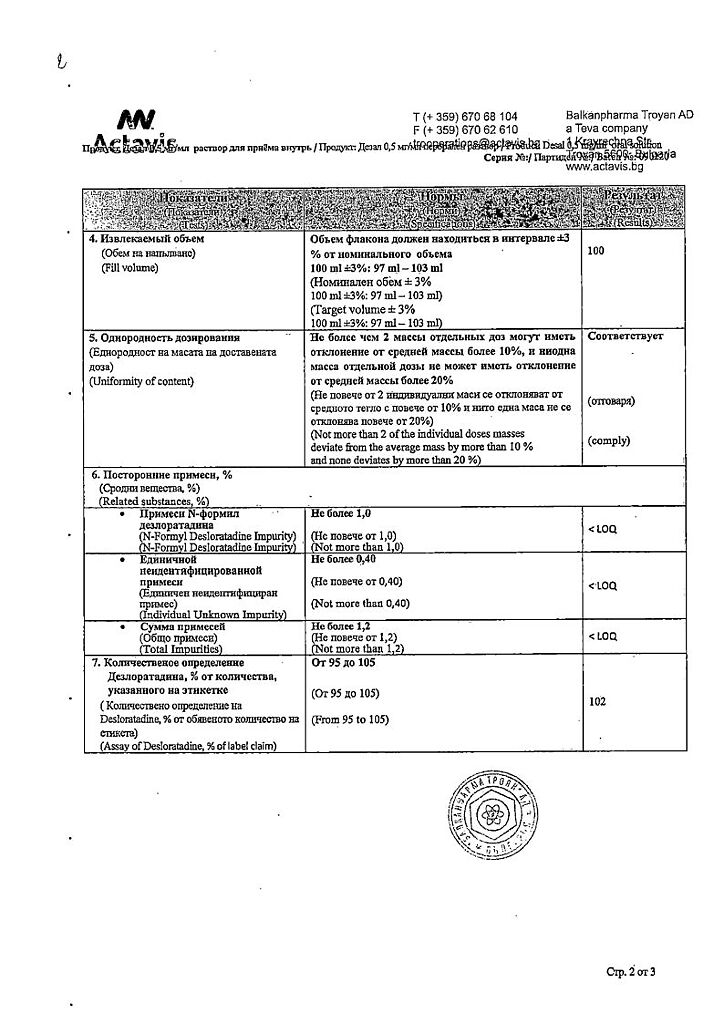
the active substance: desloratadine – 0.50 mg;
excipients: sorbitol – 147.15 mg, propylene glycol – 102.30 mg, citric acid monohydrate – 21.06 mg, sodium citrate dihydrate – 16.38 mg, hypromellose 2910 – 2.00 mg, sucralose – 1.00 mg, edetate disodium – 0.04 mg, tutti-frutti flavoring – 0.03 mg.
How to take, the dosage
How to take, the dosage
Adults and adolescents aged 12 years and older are prescribed orally, regardless of meals, in a dose of 5 mg per day.
In children aged 1 to 5 years, 1.25 mg once daily; aged 6 to 11 years, 2.5 mg once daily.
Special Instructions
Special Instructions
In cases of marked renal dysfunction, Desal should be taken with caution (see section “Pharmacokinetics”).
The differential diagnosis between allergic rhinitis and rhinitis of other origin in children under 2 years of age is difficult. In making the differential diagnosis, attention should be paid to the presence or absence of foci of infection or structural abnormalities of the upper respiratory tract, a thorough history of examination, and appropriate laboratory tests and skin tests.
In approximately 6% of adults and children aged 2 to 11 years, desloratadine’s low metabolism capacity has a higher exposure in this patient group (see Pharmacokinetics section).
The safety profile of desloratadine in children aged 2 to 11 years with low metabolism is similar to that of children with normal desloratadine metabolism. The effect of desloratadine in children younger than 2 years of age with low metabolism has not been studied.
The efficacy of desloratadine in rhinitis of infectious etiology has not been studied.
Contraindications
Contraindications
Hypersensitivity, pregnancy, breast-feeding.
For syrup (additionally, due to the presence of sucrose and sorbitol): hereditary fructose intolerance, glucose/galactose malabsorption or sucrose/isomaltose deficiency.
Side effects
Side effects
In clinical trials of desloratadine in a dosage form for oral administration 246 patients aged 6 months to 11 years were taken. In children aged from 2 to 11 years when using desloratadine the frequency of side effects was the same as when using placebo. In infants and young children (from 6 to 23 months) the following adverse events were observed while using desloratadine with a slightly higher frequency than when using placebo: diarrhea (37%) increased body temperature (23%) insomnia (23%). In an additional study no adverse events were observed in patients aged 6 to 11 years old when using desloratadine in a dose of 25 mg as an oral solution.
In clinical studies when desloratadine was administered to adults and adolescents according to indications including allergic rhinitis and chronic idiopathic urticaria, adverse reactions were reported in 3% of patients, which exceeded the similar value in the placebo group. The most frequently reported adverse reactions were the following: increased fatigue (12%) dry mouth (08%) headache (06%).
In clinical studies involving 578 adolescents 12 to 17 years old, headache was the most commonly reported headache. It occurred in 59% of patients taking desloratadine and 69% of patients taking placebo.
The incidence of adverse reactions identified in the clinical study of desloratadine exceeded that of placebo, and other adverse reactions reported in the post-registration follow-up period are listed below.
The incidence of adverse events was classified as follows: very common (â¥1/10) common (â¥1/100 < 1/10) infrequent (â¥1/1000 < 1/100) rare (â¥1/10 000 < 1/1000) very rare (< 1/10000 including individual cases) frequency unknown (cannot be estimated based on available data).
Psychiatric disorders: very rare – hallucinations.
Nervous system disorders:often – headache insomnia (for children younger than 2 years); very rarely – dizziness drowsiness insomnia psychomotor hyperactivity seizures.
Heart: very rare – tachycardia, palpitations; frequency unknown – prolongation of the QT interval.
Gastrointestinal tract: frequent – dry mouth diarrhea (in children under 2 years of age); very rare – abdominal pain nausea vomiting dyspepsia diarrhea.
Hepatic and biliary tract disorders: very rare – increased liver enzyme activity, increased bilirubin concentration hepatitis; frequency unknown – jaundice.
Skin and subcutaneous tissue disorders: frequency unknown – photosensitization.
Muscular system and connective tissue: very rare – myalgia.
General disorders and disorders at the site of administration:often – increased fatigue increased body temperature (for children younger than 2 years); very rare – hypersensitivity reactions (such as anaphylaxis angioedema dyspnea itching rash urticaria); frequency unknown – asthenia.
Post-registration period
Childhood: incidence unknown – prolongation of the QT interval arrhythmia bradycardia.
If any of the side effects listed in the instructions worsen or if you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
Overdose
Symptoms: ingestion of 9 times the recommended dose (45 mg) did not result in any clinically significant symptoms.
Treatment: gastric lavage, administration of activated charcoal; if necessary – symptomatic therapy. Desloratadine is not excreted by hemodialysis the effectiveness of peritoneal dialysis has not been established.
In case of accidental ingestion of large amounts of the drug it is necessary to consult a physician immediately.
Pregnancy use
Pregnancy use
The use of the drug during pregnancy is contraindicated due to the lack of clinical data on the safety of its use during this period.
Desloratadine is excreted into the breast milk, so its use during breast-feeding is contraindicated.
Similarities
Similarities
Additional information
| Weight | 0.210 kg |
|---|---|
| Shelf life | 2 years |
| Conditions of storage | Store in a dry, protected from light, out of the reach of children at a temperature not exceeding 25 ° C. |
| Manufacturer | Balkanpharma – Troyan AD, Bulgaria |
| Medication form | oral solution |
| Brand | Balkanpharma – Troyan AD |
Other forms…
Related products
Buy Dezal for oral administration 0.5 mg/ml, 100 ml with delivery to USA, UK, Europe and over 120 other countries.