No products in the cart.
Description
Pharmacotherapeutic group: anti-allergic agent – H1-histamine receptor blocker
ATX code: R06AX27
Pharmacological properties
Pharmacodynamics
A long-acting antihistamine drug, blocker of peripheral H1-histamine receptors. Desloratadine is the primary active metabolite of loratadine. It inhibits the cascade of allergic inflammatory reactions, including release of pro-inflammatory cytokines, including interleukins IL-4, IL-6, IL-8, IL-13, release of pro-inflammatory chemokines, production of superoxide anions by activated polymorphonuclear neutrophils, eosinophil adhesion and chemotaxis, release of adhesion molecules, such as P-selectin, IgE – mediated release of histamine, prostaglandin D2 and leukotriene C4. Thus, it prevents and facilitates the development of allergic reactions, has antipruritic and antiexudative effects, reduces capillary permeability, prevents the development of tissue edema, smooth muscle spasm.
The drug has no effect on the central nervous system, it has practically no sedative effect (does not cause sleepiness) and does not affect the speed of psychomotor reactions when taken in recommended doses. In clinical and pharmacological studies of desloratadine in the recommended therapeutic dose no prolongation of the QT interval on the electrocardiogram was observed.
The action of desloratadine starts within 30 minutes after oral administration and lasts for 24 hours.
Pharmacokinetics
absorption
.p> After oral administration of the drug, desloratadine is well absorbed from the gastrointestinal tract. It is detected in blood plasma after 30 minutes, and the maximum plasma concentration (Cmax) is reached after approximately 3 hours. The bioavailability of desloratadine is dose-proportional when administered between 5 mg and 20 mg.
Distribution
The binding to plasma proteins is 83-87%. When administered in adults and adolescents for 14 days at a dose of 5 mg to 20 mg once daily there are no signs of clinically significant cumulation of desloratadine. The degree of accumulation of desloratadine is consistent with the value of the elimination half-life and the frequency of its administration once daily. Simultaneous intake of food (fatty food, high-fat breakfast food) or grapefruit juice does not affect the distribution of desloratadine (when taken at a dose of 7.5 mg once daily). Does not penetrate through the blood-brain barrier.
Metabolism
It is not an inhibitor of CYP3A4 isoenzymes in vivo and CYP2D6 in vitro and is not a substrate or inhibitor of P-glycoprotein. It is intensively metabolized in the liver by hydroxylation to form 3-hydroxydesloratadine, which is then glucuronized.
Elimation
The terminal phase of the half-life is about 27 h. Only a small portion of the oral dose is excreted by the kidneys (less than 2%) and through the intestine (less than 7%).
Indications
Indications
To relieve or eliminate symptoms:
– allergic rhinitis (sneezing, nasal congestion, rhinorrhea, itchy nose, itchy palate, itching and redness of the eyes, lacrimation);
– urticaria (skin itching, rash).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antiallergic agent – H1-histamine receptor blocker
ATX code: R06AX27
Pharmacological properties
Pharmacodynamics
Long-acting antihistamine, peripheral H1-histamine receptor blocker. Desloratadine is the primary active metabolite of loratadine. Inhibits the cascade of allergic inflammation reactions, incl. release of proinflammatory cytokines, including interleukins IL-4, IL-6, IL-8, IL-13, release of proinflammatory chemokines, production of superoxide anions by activated polymorphonuclear neutrophils, adhesion and chemotaxis of eosinophils, release of adhesion molecules such as P-selectin, IgE-mediated release of histamine, prostaglandin D2 and leukotriene C4. Thus, it prevents the development and facilitates the course of allergic reactions, has antipruritic and antiexudative effects, reduces capillary permeability, and prevents the development of tissue edema and smooth muscle spasm.
The drug has no effect on the central nervous system, has virtually no sedative effect (does not cause drowsiness) and does not affect the speed of psychomotor reactions when taken in recommended doses. In clinical and pharmacological studies of the use of desloratadine at the recommended therapeutic dose, there was no prolongation of the QT interval on the electrocardiogram.
The action of desloratadine begins within 30 minutes after oral administration and continues for 24 hours.
Pharmacokinetics
Suction
After taking the drug orally, desloratadine is well absorbed from the gastrointestinal tract. It is determined in the blood plasma after 30 minutes, and the maximum concentration in the blood plasma (Cmax) is reached after approximately 3 hours. The bioavailability of desloratadine is dose proportional when taking a dose in the range from 5 mg to 20 mg.
Distribution
The connection with blood plasma proteins is 83-87%. When used in adults and adolescents for 14 days at a dose of 5 mg to 20 mg 1 time / day, there are no signs of clinically significant accumulation of desloratadine. The degree of accumulation of desloratadine is consistent with the half-life and the frequency of its once daily use. Simultaneous ingestion of food (fatty foods, high-calorie foods for breakfast) or grapefruit juice does not affect the distribution of desloratadine (when taken at a dose of 7.5 mg 1 time / day). Does not penetrate the blood-brain barrier.
Metabolism
It is not an inhibitor of CYP3A4 in vivo and CYP2D6 in vitro and is not a substrate or inhibitor of P-glycoprotein. Extensively metabolized in the liver by hydroxylation to form 3-hydroxydesloratadine, which is then glucuronidated.
Removal
The terminal phase of the half-life is about 27 hours. Only a small part of the dose taken orally is excreted by the kidneys (less than 2%) and through the intestines (less than 7%).
Special instructions
Special instructions
In the presence of severe renal failure, desloratadine should be used with caution.
The effectiveness of desloratadine in rhinitis of infectious etiology has not been studied.
Caution should be exercised when using desloratadine in patients with a personal or family history of seizures, especially in young children who are more susceptible to developing seizures when treated with desloratadine. If seizures occur during treatment, you should immediately consult a doctor to decide whether to stop taking desloratadine.
Impact on the ability to drive vehicles and machinery
The potential for side effects such as dizziness and drowsiness should be taken into account. If the described adverse events occur, you should refrain from performing these activities.
Active ingredient
Active ingredient
Desloratadine
Composition
Composition
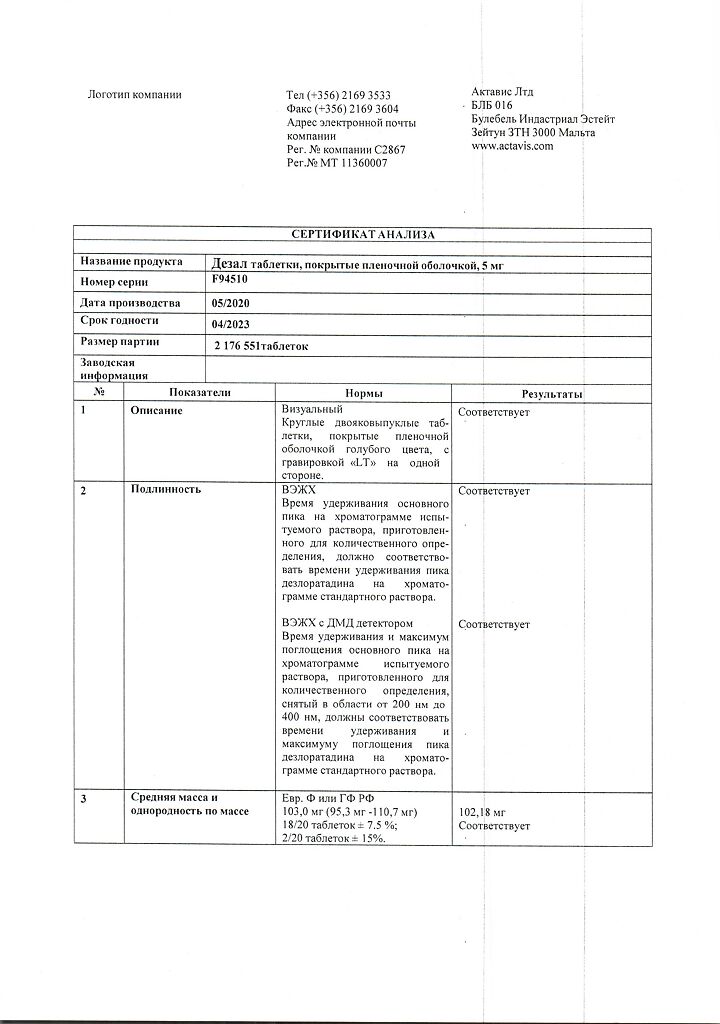
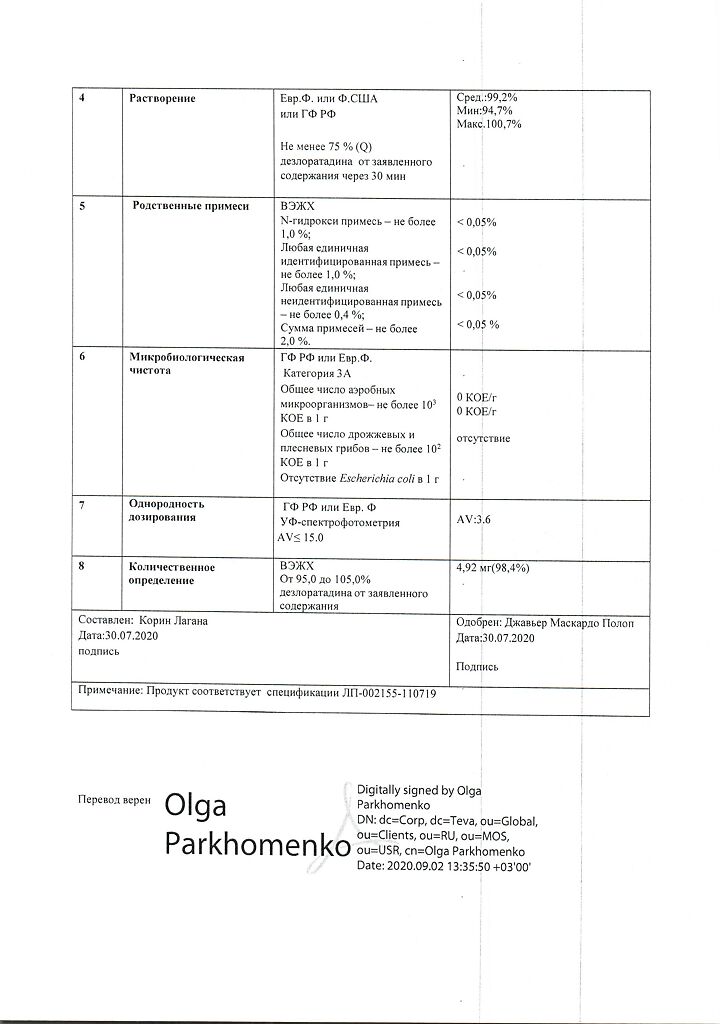
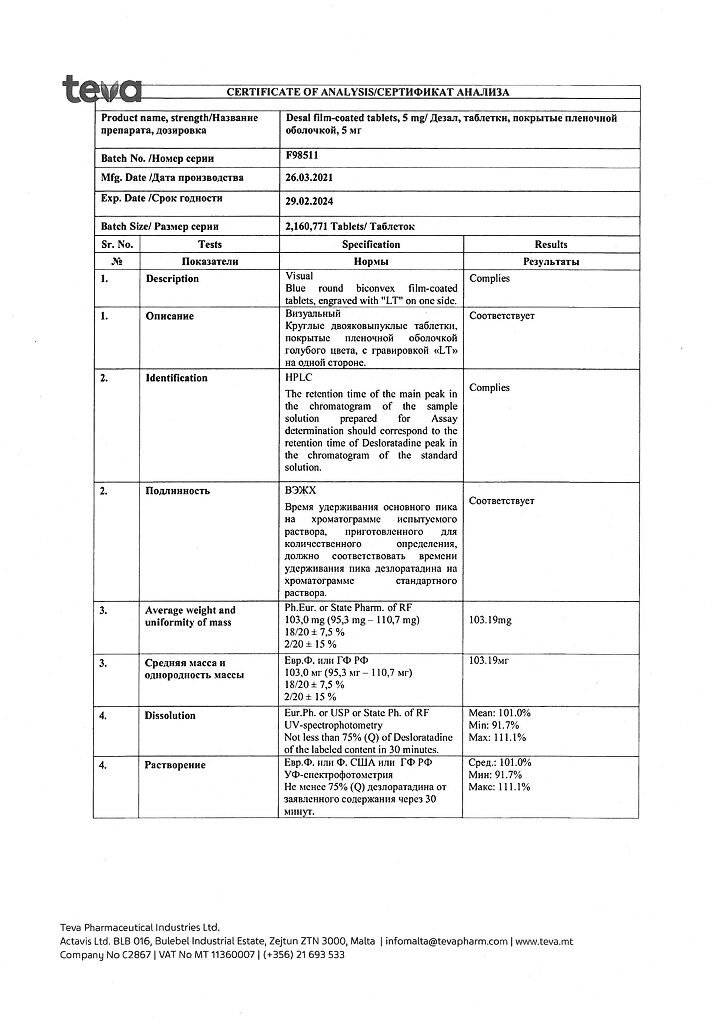
1 tablet contains:
active ingredient: desloratadine – 5.00 mg;
excipients: microcrystalline cellulose – 55.00 mg, pregelatinized corn starch – 15.00 mg, mannitol – 22.00 mg, talc – 2.50 mg, magnesium stearate – 0.50 mg;
film shell: Opadry blue 03F20404 (hypromellose 6cP – 1.90 mg, titanium dioxide (E 171) – 0.61 mg, macrogol 6000 – 0.34 mg, dye indigo carmine aluminum varnish (E 132) – 0.14 mg) about 3.00 mg.
Pregnancy
Pregnancy
The use of the drug during pregnancy is contraindicated due to the lack of clinical data on the safety of its use during this period.
Desloratadine is excreted in breast milk, so its use during breastfeeding is contraindicated.
Contraindications
Contraindications
– hypersensitivity to desloratadine, other components of the drug or loratadine;
– pregnancy and breastfeeding period;
– children under 12 years of age (efficacy and safety have not been established for this dosage form).
With caution
– severe renal failure;
– in patients with a history of seizures.
Side Effects
Side Effects
The most common side effects (≥1/100 to <1/10), the incidence of which was slightly higher than with placebo (“dummy”): fatigue (1.2%), dry mouth (0.8%) and headache (0.6%).
In children aged 12–17 years, according to the results of clinical studies, the most common side effect was headache (5.9%), the frequency of which was no higher than when taking placebo (6.9%).
Information on side effects is presented based on the results of clinical studies and observations of the post-registration period.
Adverse reactions are systematized in accordance with the World Health Organization (WHO) Classification: very common (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (< 1/10000); frequency unknown (cannot be determined from available data).
From the mental side: very rarely – hallucinations; frequency unknown – abnormal behavior, aggression.
From the nervous system: often – headache; very rarely – dizziness, drowsiness, insomnia, psychomotor hyperactivity, convulsions.
From the liver and biliary tract: very rarely – increased activity of liver enzymes, increased bilirubin concentration, hepatitis; frequency unknown – jaundice.
From the digestive system: often – dry mouth; very rarely – abdominal pain, nausea, vomiting, dyspepsia, diarrhea.
From the cardiovascular system: very rarely – tachycardia, palpitations; frequency unknown – prolongation of the QT interval.
From the musculoskeletal system: very rarely – myalgia.
From the skin and subcutaneous tissues: frequency unknown – photosensitivity.
General disorders: often – increased fatigue; very rarely – anaphylaxis, angioedema, shortness of breath, itching, rash, including urticaria; frequency unknown – asthenia.
Post-registration period
Children: frequency unknown – QT prolongation, arrhythmia, bradycardia, abnormal behavior, aggression.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
No clinically significant interactions with other drugs have been identified (including azithromycin, ketoconazole, fluoxetine, cimetidine and erythromycin). Simultaneous intake of food or grapefruit juice does not affect the effectiveness of the drug.
Desloratadine does not enhance the effect of ethanol on the central nervous system. However, cases of alcohol intolerance and alcohol intoxication have been reported during post-marketing use. Therefore, desloratadine should be used concomitantly with alcohol with caution.
Childhood
Interaction studies with desloratadine have been conducted in adult patients only.
Overdose
Overdose
Symptoms: Taking a dose 9 times higher than the recommended dose (45 mg) did not lead to the appearance of any clinically significant symptoms.
Treatment: gastric lavage is necessary, taking activated charcoal; if necessary, symptomatic therapy. Desloratadine is not eliminated by hemodialysis; the effectiveness of peritoneal dialysis has not been established.
If you accidentally ingest a large amount of the drug, you should immediately consult a doctor.
Storage conditions
Storage conditions
Store in a dry place, protected from light, at a temperature not exceeding 25 ºС. Keep out of the reach of children.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Actavis Ltd, Malta
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | Store in a dry, light-protected place at a temperature not exceeding 25 ºC. Keep out of reach of children. |
| Manufacturer | Actavis Ltd, Malta |
| Medication form | pills |
| Brand | Actavis Ltd |
Other forms…
Related products
Buy Dezal, 50 mg+450 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.