No products in the cart.
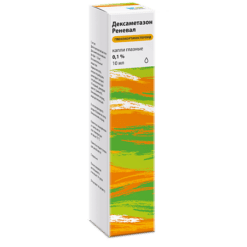
Dexamethasone, eye drops 0.1% 10 ml
€6.81 €5.96
Description
The fluorinated glucocorticosteroid has a pronounced anti-inflammatory anti-allergic and anti-exudative effect.
Interacting with a specific protein receptor in the target tissues it regulates the expression of corticoid-dependent genes and affects protein synthesis. It decreases the formation of release and activity of inflammatory mediators (histamine kinin prostaglandin lysosomal enzymes).
Inhibits cell migration to the site of inflammation; decreases vasodilation and increased vascular permeability in the inflammation focus. Stabilizes lysosomal enzymes in leukocyte membranes, inhibits antibody synthesis and disrupts antigen recognition. Inhibits the release of interleukin-1 and interleukin-2 gamma interferon from lymphocytes and macrophages. Induces the formation of lipocortin inhibits the release of inflammatory mediators by eosinophils and stabilizes mast cell membranes. All these effects are involved in suppressing the inflammatory reaction in tissues in response to mechanical chemical or immune damage.
The duration of the anti-inflammatory effect after 1 drop of the solution is from 4 to 8 hours.
Pharmacokinetics:
After injection into the conjunctival sac, it penetrates well into the corneal epithelium and conjunctiva while reaching therapeutic concentrations in the aqueous humor of the eye. If the mucous membrane is inflamed or damaged, the rate of penetration increases.
About 60-70% of dexamethasone entering the systemic bloodstream is bound to plasma proteins. It is metabolized in the liver under the action of cytochrome-containing enzymes. Metabolites are excreted through the intestine. The half-life (T1/2) of eye drops is about 3 hours.
Indications
Indications
Acute and chronic allergic and inflammatory eye diseases (including conjunctivitis, scleritis, deep keratitis without epithelial damage, iritis, iridocyclitis, chorioiditis, chorioretinitis, optic neuritis); prevention and treatment of inflammatory processes in postoperative and posttraumatic periods.
Active ingredient
Active ingredient
Composition
Composition
1 ml of the solution contains:
dexamethasone sodium phosphate 1 mg in terms of 100% dry matter;
excipients: boric acid 15 mg, sodium tetraborate decahydrate (borax) 0.6 mg, disodium edetate dihydrate 0.5 mg, benzalkonium chloride in terms of 100% dry substance 0.04 mg, water for injection to 1 ml.
How to take, the dosage
How to take, the dosage
In the conjunctival sac.
In case of pronounced inflammatory process during the first 24-48 hours of treatment 1-2 drops every hour, when the inflammatory process subsides – every 2-4 hours. Then the dose is reduced to 1 drop 3-4 times a day.
In chronic diseases 1-2 drops in the conjunctival sac of the affected eye every 3-6 hours or more frequently until the desired effect is achieved.
In allergic conditions, 1-2 drops every 3 to 4 hours until desired effect is achieved.
The course of treatment is chosen individually, on average 1-1.5 weeks.
Interaction
Interaction
Special studies of drug interactions with other medicinal products have not been conducted.
There have been reports of interaction of the active substance after systemic use. However, after injection of the eye drops the systemic absorption of dexamethasone is so low that the risk of any interaction is minimal.
If other topical ophthalmic medications are used as adjunctive therapy with the drug, a 10-15 minute interval between applications should be maintained.
Long-term use with iodoxuridine may increase destructive processes in the corneal epithelium.
Special Instructions
Special Instructions
Prolonged use of corticosteroids or increased frequency of administration may result in ocular hypertension/glaucoma with optic nerve damage and worsened visual acuity and visual field loss, and formation of subcapsular posterior chamber cataracts.
The intraocular pressure may increase in sensitive patients after using conventional doses. Continuous monitoring of intraocular pressure is recommended.
In glaucoma patients, treatment with the drug should be limited to two weeks unless prolonged treatment is warranted. Intraocular pressure should be monitored continuously.
Long-term use of steroids can develop:
Treatment should not be discontinued prematurely, as abrupt discontinuation of topical treatment with high doses of steroids can cause recurrent inflammation of the eye.
The eye drops contain the preservative benzalkonium chloride which can irritate the eyes and discolor soft contact lenses. Therefore, patients should remove their contact lenses before using Farmadex and should be informed to wait 15 minutes after instillation of Farmadex before inserting contact lenses.
Safety of use has not been investigated in patients who have renal or hepatic disease. However, because of the low systemic absorption of dexamethasone, no dose adjustment is necessary after topical administration of this medication.
The ability to affect reaction speed when driving or operating machinery.
Temporary blurred vision or other visual impairment may affect the ability to operate motor vehicles or machinery. If blurred vision occurs while injecting, the patient should wait until vision is restored before driving or operating other machinery.
Contraindications
Contraindications
Hypersensitivity in children (under 18 years of age). Viral and fungal eye diseases purulent eye infection (without concomitant antimicrobial therapy) trachoma glaucoma corneal epithelial integrity damage (including condition after removal of corneal foreign body) eye tuberculosis.
Side effects
Side effects
Visual organ disorders: possible short-term burning sensation, discomfort in the eye area, itching, tingling, hyperemia, lacrimation, local allergic reactions, swelling and itching of eyelids, possible decrease in visual acuity, visual field disturbance, increased intraocular pressure, corneal perforation (with its thinning), mydriasis, keratitis, ptosis, photophobia, formation of posterior subcapsular cataract, glaucoma, glaucoma with optic nerve damage.
In treatment with glucocorticoids, bacterial, fungal or viral (Herpes simplex) eye infections, blepharitis or conjunctivitis associated with suppression of the body’s response to infection may develop.
Overdose
Overdose
There have been no reports of severe systemic reactions due to overdose of the drug.
In case of local overdose, flush the eye(s) with warm water.
There is no specific antidote for topical use of corticosteroids. The treatment is symptomatic.
Pregnancy use
Pregnancy use
The safety and effectiveness of the drug Pharmadex for the treatment of children has not been established, so the drug is not prescribed for children.
The safety of the drug during pregnancy and lactation has not been established. Systemic manifestation is low with topical use of the eye drops, so the risk is considered small, but the benefit/risk ratio should be carefully evaluated when prescribing to pregnant and breastfeeding women.
Similarities
Similarities
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Farmak, Ukraine |
| Medication form | eye drops |
| Brand | Farmak |
Other forms…
Related products
Buy Dexamethasone, eye drops 0.1% 10 ml with delivery to USA, UK, Europe and over 120 other countries.