No products in the cart.
Dancil, eye and ear drops 0.3% 5 ml
€4.97 €4.42
Description
Dancil is an antimicrobial agent. It belongs to the group of fluoroquinolones of broad spectrum. Bactericidal effect of Ofloxacin is associated with blockade of DNA-gyrase enzyme in bacterial cells.
Highly active against most gram-negative bacteria: Escherichia coli, Salmonella spp., Shigella spp., Proteus spp., Morganella morganii, Klebsiella spp. (including Klebsiella pneumoniae), Enterobacter spp, Serratia spp., Citrobacter spp., Yersinia spp., Providencia spp., Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitidis, Mycoplasma spp., Legionella pneumophila, Acinetobacter spp. and Chlamydia spp.
Active against some Gram-positive microorganisms (including Staphylococcus spp., Streptococcus spp. /especially beta-hemolytic streptococci/).
Ofloxacin is moderately sensitive to Enterococcus faecalis, Streptococcus pneumoniae, Pseudomonas spp.
The anaerobic bacteria (except Bacteroides ureolyticus) are not sensitive to ofloxacin.
Resistant to the action of β-lactamases.
Pharmacokinetics
After oral administration, it is quickly and completely absorbed from the gastrointestinal tract. Food intake has little effect on the degree of absorption, but may slow down its rate. Cmax in plasma is reached after 2 hours.
Protein binding is 25%. Ofloxacin is widely distributed in the tissues and body fluids (organs of the urinary system, genital organs, prostate, lungs, ENT organs, gall bladder, bones, skin).
It is excreted unchanged in the urine (about 80% within 24 hours). Urinary concentrations of ofloxacin were significantly higher than MPK90 for most microorganisms after the last dose (300 mg twice daily for 14 days).
A small part of the active substance (about 4%) is excreted in the feces. T1/2 is 6 hours. In elderly patients with an average CK of 50 ml/min may increase T1/2 to 13.3 h.
Indications
Indications
Infectious and inflammatory diseases caused by microorganisms sensitive to ofloxacin: diseases of the lower respiratory tract, ear, throat, nose, skin, soft tissues, bones, joints, infectious and inflammatory diseases of the abdominal organs (with the exception of bacterial enteritis) and pelvis, kidney and urinary tract infections, prostatitis, gonorrhea.
Pharmacological effect
Pharmacological effect
Dancil is an antimicrobial agent. Belongs to the group of broad-spectrum fluoroquinolones. The bactericidal effect of ofloxacin is associated with blockade of the DNA gyrase enzyme in bacterial cells.
Highly active against most gram-negative bacteria: Escherichia coli, Salmonella spp., Shigella spp., Proteus spp., Morganella morganii, Klebsiella spp. (including Klebsiella pneumoniae), Enterobacter spp., Serratia spp., Citrobacter spp., Yersinia spp., Providencia spp., Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitidis, Mycoplasma spp., Legionella pneumophila, Acinetobacter spp., and Chlamydia spp.
Active against some gram-positive microorganisms (including Staphylococcus spp., Streptococcus spp. /especially beta-hemolytic streptococci).
Enterococcus faecalis, Streptococcus pneumoniae, Pseudomonas spp. are moderately sensitive to ofloxacin.
Anaerobic bacteria (except Bacteroides ureolyticus) are not sensitive to ofloxacin.
Resistant to β-lactamases.
Pharmacokinetics
After oral administration, it is quickly and completely absorbed from the gastrointestinal tract. Food intake has little effect on the extent of absorption, but may slow its rate. Cmax in blood plasma is reached after 2 hours.
Protein binding – 25%. Ofloxacin is widely distributed in tissues and body fluids (urinary system, genital organs, prostate gland, lungs, ENT organs, gall bladder, bones, skin).
It is excreted unchanged in the urine (about 80% in 24 hours). Concentrations of ofloxacin in urine significantly exceeded the MIC90 for most microorganisms after the last dose (300 mg 2 times a day for 14 days).
A small part of the active substance (about 4%) is excreted in the feces. T1/2 is 6 hours. In elderly patients with CC on average 50 ml/min, T1/2 may increase to 13.3 hours.
Special instructions
Special instructions
Use with caution in patients with impaired renal and hepatic function.
During treatment, it is necessary to monitor blood glucose levels. With long-term therapy, it is necessary to periodically monitor the functions of the kidneys, liver, and peripheral blood picture.
When using ofloxacin, the body should be sufficiently hydrated and the patient should not be exposed to ultraviolet radiation.
Experimental studies have not revealed any mutagenic potential. Long-term studies to determine the carcinogenicity of ofloxacin have not been conducted.
In studies in juvenile animals of several species, ofloxacin caused arthropathy and osteochondrosis.
Safety and effectiveness in children and adolescents under 18 years of age have not been established.
Impact on the ability to drive vehicles and operate machinery
Use with caution in patients whose activities require a high concentration of attention and speed of psychomotor reactions.
Active ingredient
Active ingredient
Ofloxacin
Composition
Composition
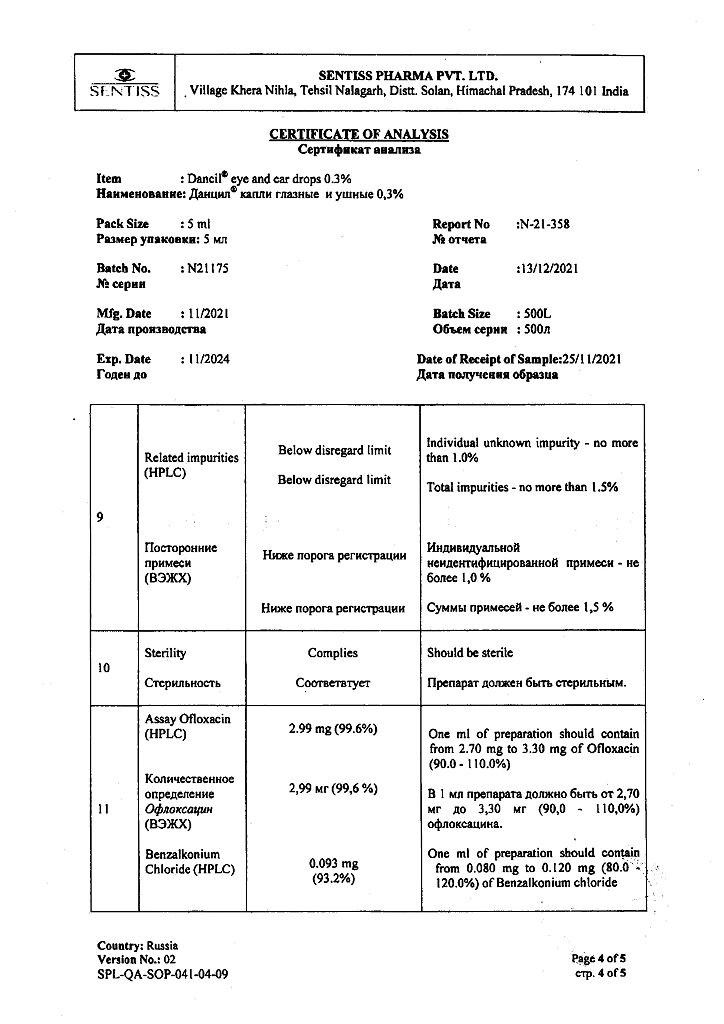
1 ml – ofloxacin 3 mg
Excipients:
benzalkonium chloride – 0.1 mg;
sodium chloride – 8 mg;
disodium edetate – 1 mg;
hypromellose – 2 mg;
sodium hydroxide – q.s.;
hydrochloric acid – q.s.;
water for injection – up to 1 ml.
Pregnancy
Pregnancy
Use during pregnancy is possible (including in the form of dosage forms for topical use) if the expected effect of therapy exceeds the potential risk to the fetus (adequate and strictly controlled studies of the safety of use in pregnant women have not been conducted).
Teratogenic effects. Ofloxacin did not have a teratogenic effect when administered to pregnant animals during the period of organogenesis: to rats in doses above 810 mg/kg/day, which is 11 times higher than the MRDC when administered orally and 9000 times when used in the form of eye drops; rabbits in doses above 160 mg/kg/day, which exceeds the MRDC by 4 and 1800 times, respectively.
Doses equivalent to 50 and 10 MRPH when taken orally were fetotoxic – a decrease in fetal body weight and an increase in fetal mortality in rats and rabbits was observed.
FDA category of effect on the fetus is C.
With a single dose of 200 mg of ofloxacin to nursing women, its concentrations in breast milk are similar to those in plasma.
Because ofloxacin has the potential to cause serious adverse reactions in breastfed infants, nursing women should discontinue either breastfeeding or ofloxacin (given the maternal significance of the drug).
Contraindications
Contraindications
hypersensitivity to ofloxacin or other quinolone derivatives.
pregnancy and lactation.
children and adolescents up to 18 years of age.
Side Effects
Side Effects
From the gastrointestinal tract:
nausea, vomiting, diarrhea, abdominal pain and cramps, loss of appetite, dry mouth, flatulence, gastrointestinal dysfunction, constipation;
rarely – liver dysfunction, liver necrosis, jaundice, hepatitis, intestinal perforation, pseudomembranous colitis, bleeding from the gastrointestinal tract, disorders of the oral mucosa, heartburn, increased activity of liver enzymes, including GGT and LDH, increased bilirubin levels in the blood serum.
From the central and peripheral nervous system:
insomnia, dizziness, fatigue, drowsiness, nervousness;
rarely – convulsions, anxiety, cognitive changes, depression, pathological dreams, euphoria, hallucinations, paresthesia, syncope, tremor, confusion, nystagmus, suicidal thoughts or attempts, disorientation, psychotic reactions, paranoia, phobia, agitation, aggressiveness, emotional lability, peripheral neuropathy, ataxia, coordination disorders, exacerbation of extrapyramidal disorders, speech impairment.
Allergic reactions:
skin rash, itching;
rarely – angioedema, urticaria, vasculitis, allergic pneumonitis, anaphylactic shock, erythema multiforme, Stevens-Johnson syndrome, erythema nodosum, exfoliative dermatitis, toxic epidermal necrolysis, conjunctivitis.
From the reproductive system:
itching in the area of the external genitalia in women, vaginitis, vaginal discharge;
rarely – burning, irritation, pain and rash in the genital area in women, dysmenorrhea, menorrhagia, metrorrhagia, vaginal candidiasis.
From the side of metabolism:
rarely – thirst, weight loss, hyper- or hypoglycemia (especially in patients with diabetes mellitus receiving insulin or oral hypoglycemic agents), acidosis, increase in serum TG, cholesterol, potassium.
From the respiratory system:
rarely – cough, nasal discharge, respiratory arrest, dyspnea, bronchospasm, stridor.
From the cardiovascular system:
rarely – cardiac arrest, edema, arterial hypertension, arterial hypotension, palpitation, vasodilation, cerebral thrombosis, pulmonary edema, tachycardia.
From the urinary system:
rarely – dysuria, increased frequency of urination, urinary retention, anuria, polyuria, kidney stone formation, renal failure, nephritis, hematuria, albuminuria, candiduria.
From the musculoskeletal system:
rarely – arthralgia, myalgia, tendonitis, muscle weakness, exacerbation of myasthenia gravis.
From the senses:
rarely – hearing impairment, tinnitus, diplopia, nystagmus, impaired clarity of visual perception, disturbances of taste, smell, photophobia.
Dermatological reactions:
rarely – photosensitivity, hyperpigmentation, vesiculobullous rashes.
From the hematopoietic system:
rarely – anemia, bleeding, pancytopenia, agranulocytosis, leukopenia, reversible inhibition of bone marrow hematopoiesis, thrombocytopenia, thrombocytopenic purpura, petechiae, ecchymosis, increased prothrombin time.
Other: chest pain, pharyngitis, fever, body pain; rarely – asthenia, chills, general malaise, nosebleeds, increased sweating.
Interaction
Interaction
When used simultaneously with antacids containing calcium, magnesium or aluminum, with sucralfate, with drugs containing di- and trivalent cations such as iron, or with multivitamins containing zinc, the absorption of quinolones may be impaired, leading to a decrease in their concentration in the body. These drugs should not be used within 2 hours before or within 2 hours after taking ofloxacin.
With the simultaneous use of ofloxacin and NSAIDs, the risk of developing a stimulating effect on the central nervous system and seizures increases.
When used simultaneously with theophylline, it is possible to increase its concentration in the blood plasma (including at steady state), and increase the half-life. This increases the risk of developing adverse reactions associated with theophylline.
With simultaneous use of ofloxacin with beta-lactam antibiotics, aminoglycosides and metronidazole, additive interactions were observed.
Overdose
Overdose
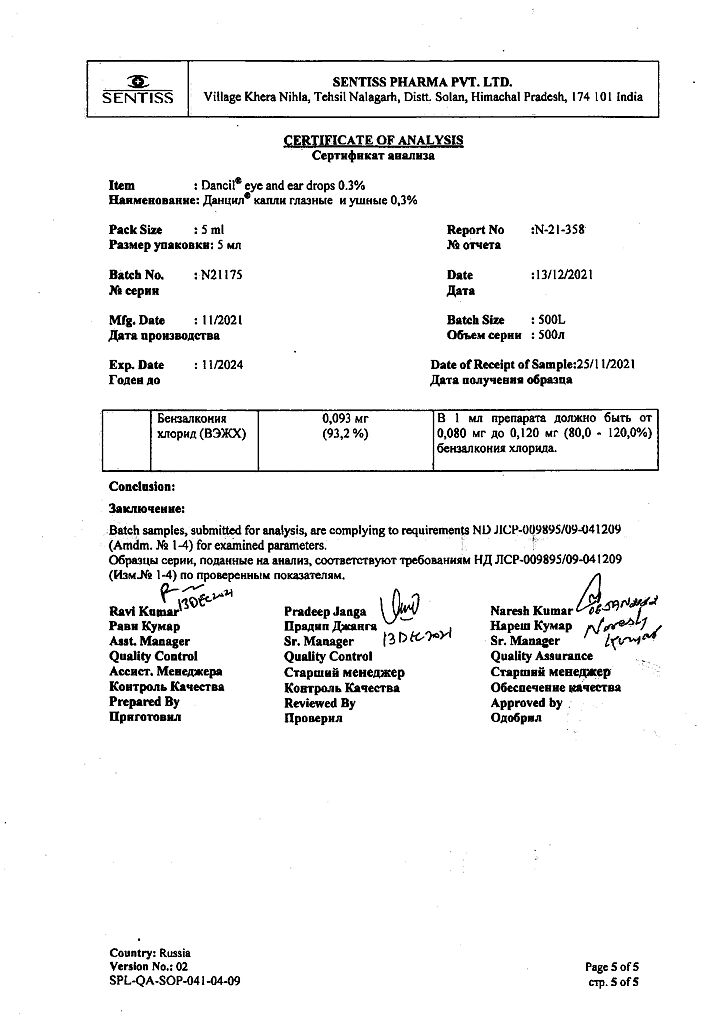
After topical application of an excess dose of Dancil, the eyes should be rinsed with clean water at room temperature.
There are no data on systemic manifestations of overdose.
Storage conditions
Storage conditions
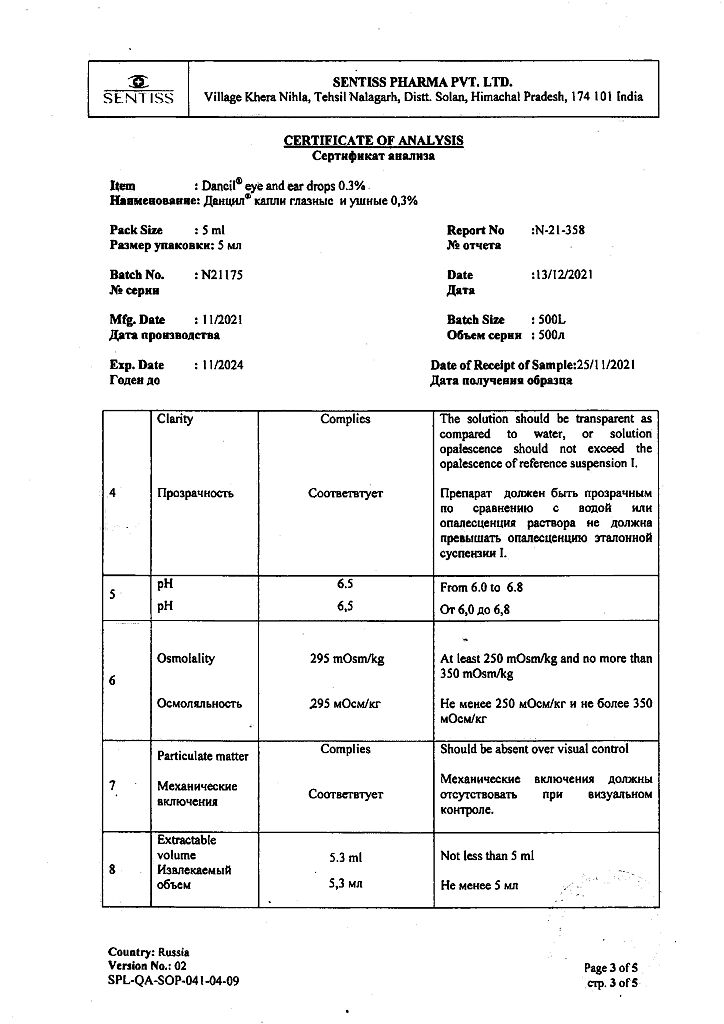
In a place protected from light, at a temperature not exceeding 25 °C (do not freeze)
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Sentiss Pharma Pvt.Ltd, India
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 25 °C (do not freeze) |
| Manufacturer | Sentiss Pharma Pvt.Ltd, India |
| Medication form | eye and ear drops |
| Brand | Sentiss Pharma Pvt.Ltd |
Related products
Buy Dancil, eye and ear drops 0.3% 5 ml with delivery to USA, UK, Europe and over 120 other countries.