No products in the cart.
Corinfar Retard, 20 mg 50 pcs
€4.84 €4.31
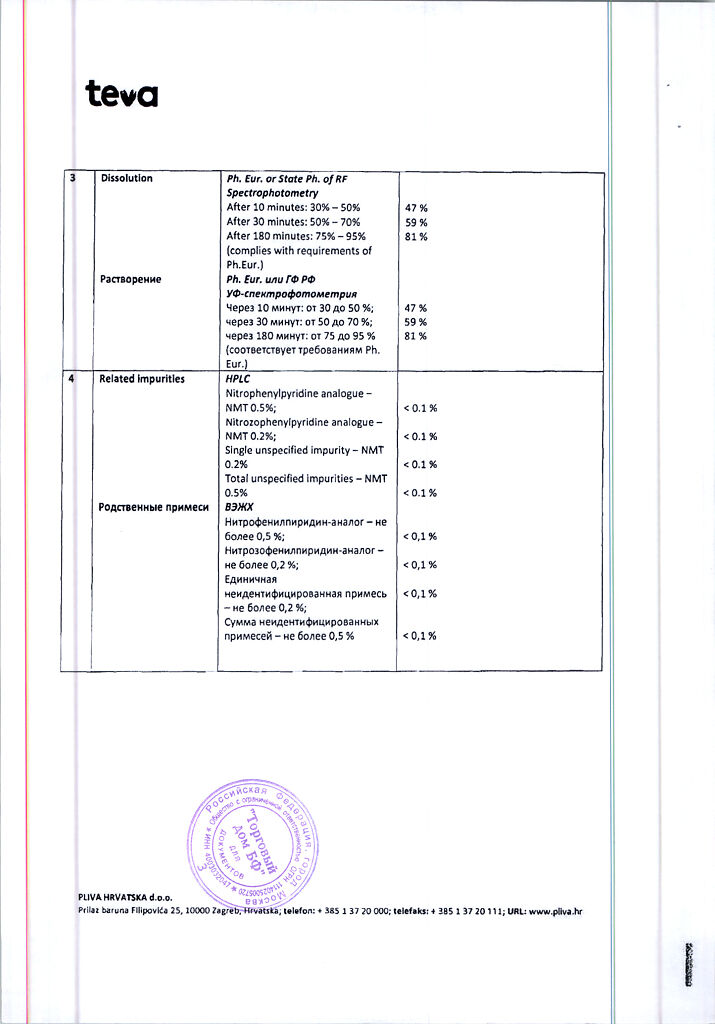
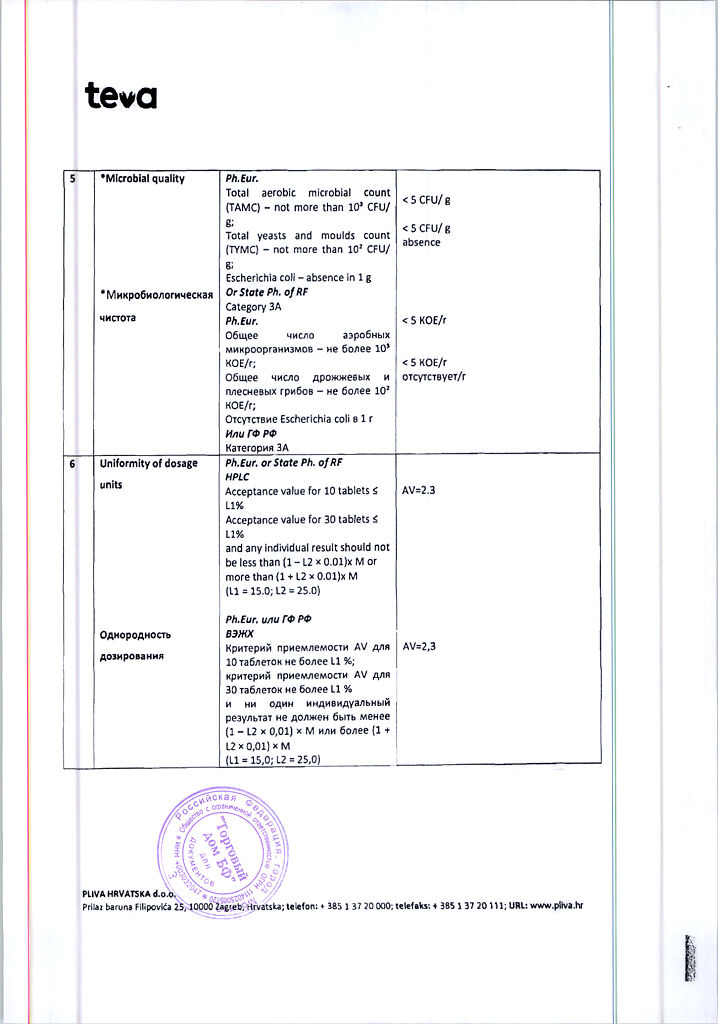
Description
Pharmacotherapeutic group: slow calcium channel blockers
ATX code: C08CA05
Pharmacological action
Pharmacodynamics
Nifedipine is a selective slow calcium channel blocker (BMCC), a 1,4-dihydropyridine derivative. It dilates coronary and peripheral arteries, reduces myocardial oxygen demand by reducing the post-load on the heart and oxygen delivery. Increases coronary blood flow, improves blood supply to ischemic areas without development of the “bypass” phenomenon, activates collaterals. By dilating peripheral arteries, it reduces total peripheral vascular resistance (TPR), myocardial tone, afterload, oxygen demand and increases duration of left ventricular (LV) diastolic relaxation. It has almost no effect on sinoatrial and atrioventricular nodes. Does not have antiarrhythmic and proarrhythmic effects. Does not affect the tone of the veins. Nifedipine increases renal blood flow, causes moderate natriuresis.
It has negative chrono-, dromo- and inotropic effects accompanied by reflex activation of the sympathoadrenal system and increased heart rate (HR) in response to peripheral vasodilation. Predominantly at the beginning of therapy, heart rate and cardiac output may decrease as a result of baroreceptor reflex activation. With prolonged therapy with nifedipine, heart rate and cardiac output return to the values they had before the start of therapy.
Nifedipine has antihypertensive and antianginal effects.
In arterial hypertension, the drug reduces blood pressure (BP) due to peripheral vasodilation and reduction of PPS. Nifedipine once daily provides 24-hour control of elevated BP. In patients with normal BP, nifedipine has little or no effect on BP.
In angina, nifedipine reduces peripheral and coronary vascular resistance, resulting in increased coronary blood flow, cardiac output and stroke volume, and decreased afterload. In addition, nifedipine dilates both intact and atherosclerotically altered coronary arteries, prevents coronary artery spasm, and improves perfusion of ischemic myocardium. Nifedipine reduces the frequency of angina attacks and ischemic ECG changes, regardless of whether they are caused by coronary artery spasm or atherosclerosis.
Pharmacokinetics
Absorption – high (more than 90%). Bioavailability is 50-70%. Simultaneous intake of food does not affect absorption. It has the effect of “first passage” through the liver.
Distribution. It penetrates through the blood-brain and placental barriers and is excreted with breast milk. Binding with blood plasma proteins (albumin) is 95%. Metabolism. It is completely metabolized in the liver. The metabolism of nifedipine is mainly carried out by the CYP3A4 isoenzyme, but also by the CYP1A2 and CYP2A6 isoenzymes. Evolution. There is no cumulative effect. Chronic renal failure, hemodialysis and peritoneal dialysis have no effect on pharmacokinetics. In patients with hepatic impairment, total clearance is decreased and T1/2 is increased. Long-term use (2-3 months) develops tolerance to the effects of the drug. Patient special groups Elderly patients . When nifedipine preparations are used in the form of sustained-release/modified-release tablets, increased Cmax and T1/2 have been noted in elderly patients (age > 60 years) compared to younger patients. Patients with impaired liver function Pharmacokinetic studies have shown that patients with cirrhosis have significantly increased T1/2 and decreased total clearance of nifedipine. In patients with mild (Child-Pugh class A) and moderate (Child-Pugh class B) hepatic impairment, clearance of nifedipine after oral administration was decreased by 48% and 72%, respectively, compared to patients with normal liver function, according to a clinical study. There was observed 93% and 64% increase of area under “concentration-time” curve (AUC) and Cmax, respectively, in patients with mild hepatic impairment (Child-Pugh class A) and 253% and 171% in patients with moderate hepatic impairment (Child-Pugh class B) compared with patients with normal hepatic function. The pharmacokinetics of nifedipine have not been studied in patients with severe hepatic impairment (Child-Pugh class C). Patients with impaired renal function The elimination of nifedipine may be delayed in patients with impaired renal function. Chronic renal failure, hemodialysis and peritoneal dialysis have no significant effect on the pharmacokinetics of nifedipine. There is no cumulative effect. Children and adolescents Pharmacokinetic studies have not been performed.
Indications
Indications
Corinfar® retard is used in adults to treat the following diseases:
• arterial hypertension;
• stable angina and vasospastic angina (Prinzmetal’s angina, variant angina).
If there is no improvement or you feel worse, you should consult a doctor.
Special instructions
Special instructions
Before using Corinfar® retard, consult your doctor or pharmacist.
Be sure to tell your healthcare provider if any of the following applies to you:
• if you have low blood pressure;
• if you have a significant increase in blood pressure;
• if you have coronary heart disease (especially with severe obstructive lesions of the coronary arteries) or cerebrovascular disease;
• if you have chronic heart failure;
• if you are taking any medications to treat heart disease;
• if you have aortic stenosis, mitral stenosis, hypertrophic obstructive cardiomyopathy;
• if you have diabetes;
• if you have mild liver dysfunction;
• if you have high blood pressure and are undergoing hemodialysis;
• if you are taking phenytoin, carbamazepine, phenobarbital, macrolide antibiotics, drugs for the treatment of HIV infection, ketoconazole, antidepressants, fluoxetine, valproic acid and other inhibitors and/or inducers of the CYP3A4 isoenzyme;
• if you are more than 20 weeks pregnant;
• old age.
Children and teenagers
Do not give the drug to children from 0 to 18 years of age, since the safety and effectiveness of the use of the drug Corinfar® retard in children and adolescents has not been established.
Driving vehicles and working with machinery
In some patients, especially at the beginning of treatment, nifedipine may cause dizziness, which reduces the ability to drive vehicles or use other machinery. In the future, the degree of restrictions depends on the individual tolerance of nifedipine.
During the treatment period, especially at the beginning of treatment or when changing the dose, care must be taken when driving, engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
The drug Corinfar® retard contains lactose monohydrate
If you have an intolerance to certain sugars, consult your doctor before taking this drug.
Active ingredient
Active ingredient
Nifedipine
Composition
Composition
The active ingredient is nifedipine.
Each tablet contains 20 mg of nifedipine.
Other ingredients (excipients) are lactose monohydrate, potato starch, microcrystalline cellulose, povidone K25, magnesium stearate, hypromellose, macrogol 6000, macrogol 35000, quinoline yellow dye (E104), titanium dioxide (E 171), talc.
Pregnancy
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or are planning a pregnancy, consult your doctor or pharmacist before using this drug.
Pregnancy
There are no adequate controlled studies in pregnant women. The available information is insufficient to exclude the possibility of side effects that pose a danger to the fetus and newborn.
In animal studies, nifedipine has been shown to be embryotoxic, fetotoxic and teratogenic.
Do not take Corinfar® retard if you are pregnant.
Breastfeeding
Nifedipine is excreted into breast milk.
Do not take Corinfar® retard if you are breastfeeding.
Fertility
In male patients, nifedipine may cause sperm damage. After several unsuccessful attempts at in vitro fertilization, exposure to nifedipine or similar antihypertensive agents can be assumed unless otherwise indicated. This may be the reason for unsuccessful attempts at in vitro fertilization.
Contraindications
Contraindications
Do not take Corinfar® retard:
• if you are allergic to nifedipine or any of the other ingredients of this medicine (listed in section 6 of the leaflet);
• if you have moderate or severe liver failure (Child-Pugh Classes B and C);
• if you have cardiogenic shock;
• if you have a collapse;
• if you have severe arterial hypotension (systolic blood pressure below 90 mmHg);
• if you have an acute period of myocardial infarction (during the first 4 weeks);
• if you have unstable angina;
• if you have hemodynamically significant obstruction of the left ventricular outflow tract (including severe aortic stenosis);
• if you are taking rifampicin (due to the inability to achieve effective plasma levels of nifedipine due to enzyme induction);
• if you are pregnant (up to 20 weeks);
• if you are breastfeeding.
If you think any of the following applies to you, tell your healthcare provider.
Side Effects
Side Effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
The most serious adverse reactions reported with nifedipine:
Uncommon – may occur in up to 1 in 100 people:
itching of the skin, in the eye area, runny nose, sneezing, rash, hives (allergic reaction);
swelling of the skin and mucous membranes, difficulty breathing, difficulty swallowing, soreness, cough, increasing hoarseness, respiratory failure, headache, possible convulsions, impaired consciousness (angioedema (Quincke’s edema)).
Rare – may affect up to 1 in 1,000 people:
pressing, often burning pain in the heart (behind the sternum), weakness, palpitations, pallor of the skin (manifestation of angina attacks);
frequent appearance of bruises, even after minor blows, increased bleeding time from wounds, poor regeneration of the wound surface, gingival bleeding, the appearance of nosebleeds (thrombocytopenic purpura).
Unknown – the frequency of occurrence cannot be determined based on the available data:
urticaria and swelling of the skin, breathing problems, most often reminiscent of an asthmatic attack, darkening of the eyes, dizziness, a sharp decrease in blood pressure, loss of consciousness (anaphylactic/anaphylactoid reactions);
the appearance of red spots, rashes and blisters on the skin and mucous membranes, detachment of the upper skin (toxic epidermal necrolysis (Lyell’s syndrome));
acute pain in the left half of the chest, shortness of breath, weakness, dizziness, the appearance of sticky sweat, a feeling of fear, panic attacks, heart rhythm disturbances (myocardial infarction);
dyspnea.
Stop taking Corinfar® retard and seek immediate medical attention if any of the above serious adverse reactions occur.
Other adverse reactions reported with nifedipine preparations:
Common – may occur in no more than 1 in 10 people:
headache;
weakness;
peripheral edema;
asymptomatic decrease in blood pressure, development or worsening of heart failure, “flushes” of blood to the facial skin, flushing of the facial skin, sensation of heat (manifestation of excessive dilation of blood vessels (vasodilation));
constipation;
asthenia.
Uncommon – may occur in up to 1 in 100 people:
sleep disorders, anxiety;
migraine;
visual impairment, including short-term loss of vision against the background of the maximum concentration of nifedipine in the blood plasma;
feeling of heartbeat;
marked decrease in blood pressure, fainting;
nosebleeds, nasal congestion;
pain in the abdomen (gastrointestinal and abdominal pain);
nausea, diarrhea, constipation (dyspepsia);
flatulence;
dryness of the oral mucosa;
increased activity of “liver” transaminases in the blood;
limited intense redness of the skin (erythema);
muscle cramps;
swelling of the joints;
disturbance of urination (polyuria, dysuria);
erectile dysfunction;
nonspecific pain;
chills.
Rare – may affect up to 1 in 1,000 people:
painful burning sensation (burning sensation under the skin), tingling or aching pain (paresthesia, dysesthesia);
skin itching, jaundice, constipation, bitter taste in the mouth, pain in the right hypochondrium, dark urine and discoloration of feces (intrahepatic cholestasis);
manifestation of sensitivity to sunlight in the form of a skin rash (photodermatitis);
bleeding, pain, swelling of the gums;
difficulty breathing, cough;
pain in muscles and joints (myalgia, arthralgia).
Very rare – may occur in no more than 1 person in 10,000:
anemia;
autoimmune hepatitis;
weight gain;
an increase in glucose levels in the blood serum compared to normal (hyperglycemia);
shortness of breath, including asthmatic symptoms (bronchospasm);
enlargement of the mammary gland with hypertrophy of the glands and adipose tissue in elderly patients (gynecomastia);
secretion of milk from the mammary glands, which is not associated with the process of feeding the child (galactorrhea).
Unknown – the frequency of occurrence cannot be determined based on the available data:
decreased level of leukocytes in the blood (leukopenia, agranulocytosis);
dullness of sensations, weakening of sensitivity in certain parts of the body (hypesthesia);
drowsiness;
increased fatigue;
numbness in the limbs (limb paresthesia);
depression;
parkinsonian disorders (impaired motor skills (ataxia), “mask-like” face, shuffling gait, stiffness in hand movements, tremor of the hands and fingers, difficulty swallowing);
eye pain;
arrhythmia;
pulmonary edema;
vomit;
insufficiency of the lower esophageal (gastroesophageal) sphincter;
increased appetite;
finely spotted capillary hemorrhages in the skin, under the skin or in the mucous membranes (purpura);
joint inflammation (arthritis);
deterioration of renal function in patients with renal failure.
Reporting Adverse Reactions
If you experience any unwanted reactions, consult your doctor or pharmacist. This also includes any adverse reactions not listed in the package insert.
Interaction
Interaction
Tell your doctor or pharmacist if you are taking, have recently taken, or may start taking any other medications.
Be sure to tell your doctor if you are taking any of the following medications:
• rifampicin (a drug used to treat tuberculosis);
• phenytoin (anti-epileptic drug);
• carbamazepine (anti-epileptic drug);
• phenobarbital (anti-epileptic drug);
• valproic acid (anti-epileptic drug);
• erythromycin (a drug to treat bacterial infections);
• ritonavir (a drug used to treat HIV infection);
• ketoconazole (antifungal drug);
• cimetidine (a drug for the treatment of gastric and duodenal ulcers);
• ranitidine (a drug for the treatment of gastric and duodenal ulcers);
• diltiazem (a drug to treat high blood pressure);
• fluoxetine (antidepressant);
• nefazodone (antidepressant);
• quinidine (antiarrhythmic drug);
• quinupristin/dalfopristin (a drug for the treatment of staphylococcal infections);
• cisapride (laxative);
• digoxin (a drug used to treat heart failure);
• theophylline (a drug for the treatment of broncho-obstructive syndrome);
• tacrolimus (immunosuppressant);
• vincristine (antitumor agent);
• indirect anticoagulants (coumarin and indanedione derivatives);
• anticonvulsants;
• non-steroidal anti-inflammatory drugs;
• sulfinpyrazone (a drug to treat gout);
• cephalosporins (drugs for the treatment of infectious and inflammatory diseases).
Corinfar® retard with food, drinks and alcohol
During the treatment period, it is not recommended to drink alcoholic beverages due to the risk of an excessive decrease in blood pressure.
Grapefruit juice inhibits the breakdown of nifedipine, which may increase or prolong the antihypertensive effect and cause a drop in blood pressure, so drinking grapefruit juice is not recommended while taking nifedipine. The inhibitory effect may last up to 3 days after the last dose of grapefruit juice.
Simultaneous food intake delays, but does not reduce, the absorption of the active substance from the gastrointestinal tract.
Overdose
Overdose
Symptoms
Nifedipine causes peripheral vasodilation with severe and possibly prolonged systemic arterial hypotension: headache, facial flushing, prolonged pronounced decrease in blood pressure, depression of the sinus node, bradycardia and/or tachycardia, bradyarrhythmia. In case of severe poisoning – loss of consciousness, coma.
Treatment
Treatment of overdose consists of standard procedures for removing the drug from the body. The administration of activated carbon, gastric lavage (if necessary, lavage of the small intestine), and restoration of stable hemodynamic parameters are indicated.
In case of an overdose of long-acting nifedipine preparations, it is necessary to ensure the most complete removal of the drug from the body, and, if possible, lavage of the small intestine in order to prevent further absorption of the active substance. When using laxatives, it should be taken into account that BMCC can cause a decrease in the tone of the intestinal muscles, up to intestinal atony.
The antidote to nifedipine is calcium preparations. Intravenous administration of 10-20 ml of a 10% solution of calcium chloride or calcium gluconate is indicated, followed by switching to a long-term infusion. If the administration of calcium supplements fails to achieve a sufficient increase in blood pressure, it is possible to use alpha adrenergic agonists (dopamine, norepinephrine).
For bradyarrhythmia – intravenous administration of atropine, beta-adrenergic agonists. For life-threatening bradyarrhythmias, installation of a temporary pacemaker is recommended. With the development of heart failure – intravenous administration of strophanthin. Infusion therapy is recommended to be carried out with caution due to the risk of volume overload of the heart.
Careful monitoring of the activity of the heart, lungs and excretory system is necessary. It is recommended to monitor the concentration of glucose (insulin release may decrease) and the content of electrolytes (potassium, calcium) in the blood.
Hemodialysis is ineffective due to the high degree of binding to plasma proteins and the relatively small volume of distribution. Plasmapheresis is possible.
Storage conditions
Storage conditions
Keep the drug out of the reach of children and so that the child cannot see it.
Do not use the drug after the expiration date indicated on the blister, bottle label or on the carton after the words “Best before”. The expiration date is the last day of the given month.
Store at a temperature not exceeding 25 ° C in the original packaging.
Do not throw the drug into the sewer. Ask your pharmacist how to dispose of (destroy) a drug that is no longer needed. These measures will protect the environment.
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Pliva Hrvatska d.o.o., Croatia
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | Store at a temperature not higher than 25 ° C in the original package. KEEP OUT OF REACH OF CHILDREN. |
| Manufacturer | Pliva Hrvatska d.o.o., Croatia |
| Medication form | sustained release tablets |
| Brand | Pliva Hrvatska d.o.o. |
Other forms…
Related products
Buy Corinfar Retard, 20 mg 50 pcs with delivery to USA, UK, Europe and over 120 other countries.