No products in the cart.
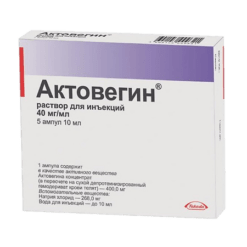
Complivit Calcium D3, orange 120 pcs
€16.39 €13.66
Description
Pharmacotherapeutic group: calcium-phosphorus metabolism regulator.
ATC code: [A12AX].
Pharmacological properties
Pharmacodynamics
Combined drug regulating calcium and phosphorus metabolism in the body (bones, teeth, nails, hair, muscles). It reduces resorption (resorption) and increases bone density, compensating the lack of calcium and vitamin D3 in the body, increases absorption of calcium in the intestine and reabsorption of phosphate in the kidneys, promotes mineralization of bones and teeth.
Calcium is involved in the formation of bone tissue, in maintaining a stable cardiac function, in the regulation of nerve conduction, muscle contractions, production of hormones and is a component of the blood clotting system.
The adequate intake of calcium is especially important during growth, pregnancy and lactation.
Vitamin D3 (colecalciferol) increases the absorption of calcium in the intestine and promotes the formation and mineralization of bone and dental tissue.
The use of calcium and vitamin D3 prevents increased production of parathyroid hormone (PTH), which is a stimulant of increased bone resorption (leaching of calcium from bone).
Pharmacokinetics.
Calcium
Intake: Calcium is absorbed in ionized form in the proximal small intestine through an active, D-vitamin dependent transport mechanism. Typically, the amount of calcium absorbed in the gastrointestinal tract is approximately 30% of the dose taken.
Distribution and metabolism: 99% of calcium in the body is concentrated in the hard structure of the bones and teeth. The remaining 1% is in intra- and extracellular fluids.
Excretion: calcium is excreted by the intestines, kidneys and sweat glands. Renal excretion depends on glomerular filtration and tubular reabsorption of calcium.
Vitamin D3
Intake: Vitamin D3 is easily absorbed in the small intestine (about 80% of the dose taken).
Distribution and metabolism: coleciferol and its metabolites circulate in the blood, bound to specific globulin. Colocalciferol is converted in the liver by hydroxylation to 25-hydroxycalciferol.
It is then converted in the kidneys to the active form 1,25-hydroxycalciferol, responsible for increasing calcium absorption in the intestine and tubular reabsorption in the kidneys. Unmetabolized vitamin D3 is deposited in fat and muscle tissue. 3 is excreted by the intestine and kidneys.
Indications
Indications
Prevention and treatment of calcium and/or vitamin D3 deficiency.
Prevention and comprehensive therapy of osteoporosis and its complications (bone fractures).
Active ingredient
Active ingredient
Composition
Composition
Active ingredients:
Calcium – 500 mg (in the form of calcium carbonate – 1.250 g).
Calciferol (vitamin D3) – 0.005 mg (200 IU) (in the form of a granule containing 0.27% colecalciferol, D,L-alpha-tocopherol – 0.0275%, medium-chain triglycerides – 10,7%, sucrose – 36%, acacia gum – 22%, corn starch – 27%, calcium phosphate (E 341) – 0,5%, water up to 100%).
Auxiliary substances: lactose monohydrate – 327.406 mg, povidone (polyvinylpyrrolidone medium molecular weight, povidone K 30) – 68.223 mg, potato starch – 20.541 mg, croscarmellose sodium – 49,875 mg, citric acid (as citric acid monohydrate) – 3.325 mg, aspartame (E 951) – 5.95 mg, magnesium stearate – 15.75 mg, orange flavoring (powder) – 8.925 mg.
How to take, the dosage
How to take, the dosage
Complivit® Calcium D3, chewable tablets, apply inally with meals. The tablet should be chewed or sucked. After that, if necessary, it can be drunk with water.
Adults: for treatment of osteoporosis – 1 tablet 2-3 times a day, to prevent osteoporosis – 1 tablet 2 times a day.
In case of calcium and/or vitamin D3 deficiency:
Adults and children over 12 years – 1 tablet 2 times a day.
Children from 5 years to 12 years – 1 to 2 tablets per day.
In children from 3 to 5 years of age, the dosage according to the doctor’s recommendation.
Special patient groups
Patients with impaired liver function:
Dose adjustment is not required.
Patients with impaired renal function:
The drug should not be used in severe renal failure.
Elderly patients:
The dose is the same as for adults. Possible decreased creatinine clearance should be considered.
The duration of treatment
When used for prevention and in complex therapy of osteoporosis the duration of treatment is determined by the doctor individually.
When used to compensate for calcium and/or vitamin D3 deficiency, the average duration of treatment is at least 4-6 weeks. The number of repeated courses during the year is determined individually.
Interaction
Interaction
Vitamin D3 activity may decrease when used concomitantly with phenytoin or barbiturates.
Hypercalcemia may potentiate toxic effects of cardiac glycosides when used simultaneously with calcium and vitamin D preparations. Monitoring of ECG and serum calcium content is necessary.
Calcium preparations may decrease absorption of tetracyclines from the gastrointestinal tract. Therefore tetracyclines should be taken at least 2 hours before or 4-6 hours after taking Complivit® Calcium D3.
In order to prevent reduced absorption of bisphosphonate preparations, it is recommended that they be taken at least one hour before taking Complivit® Calcium D3.
Glucocorticosteroids decrease calcium absorption, so treatment with glucocorticosteroids may require increasing the dose of Complivit® Calcium D3.
Concomitant treatment with colesteramine preparations or mineral or vegetable oil-based laxatives may decrease vitamin D3 absorption.
The concomitant use of thiazide-type diuretics increases the risk of hypercalcemia because they increase the tubular reabsorption of calcium. When concomitant use of thiazide diuretics, serum calcium content should be regularly monitored. In contrast, furosemide and other “loop” diuretics increase renal calcium excretion.
Calcium reduces the effectiveness of levothyroxine by reducing its absorption. The time period between levothyroxine and Complivit® Calcium D3 should be at least 4 hours.
The absorption of antibiotics of the quinolone group is reduced when used concomitantly with calcium preparations. Therefore, antibiotics of quinolone group should be taken 2 hours before or 6 hours after taking Complivit® Calcium D3.
The intake of foods containing oxalates (sorrel, rhubarb, spinach) and phytin (cereals) reduces the absorption of calcium, so you should not take Complivit.sup>® Calcium D3 within 2 hours of eating sorrel, rhubarb, spinach, and cereals.
Special Instructions
Special Instructions
Serum calcium and creatinine should be monitored during long-term therapy. Monitoring is especially important in elderly patients with concomitant treatment with cardiac glycosides and diuretics (see section “Interaction with other medicinal products and foodstuffs”) and in patients with increased propensity to form kidney stones.
In cases of hypercalcemia or signs of impaired renal function, the dose should be reduced or treatment stopped.
Vitamin D3 should be taken with caution in patients with renal insufficiency. In this case it is necessary to control the content of calcium and phosphate in serum. Also the risk of soft tissue calcinosis should be considered.
In order to avoid an overdose, additional vitamin D intake from other sources must be considered.
In the elderly, the need for calcium is 1,500 mg/day and vitamin D3 is 0.5-1,000 IU/day.
Calcium and vitamin D3 should be used with caution in immobilized patients with osteoporosis because of the risk of hypercalcemia.
Simultaneous use with antibiotics of the tetracycline or quinolone group is generally not recommended or should be used with caution (see section “Interaction with other drugs and foods”).
Impact on the ability to drive vehicles and mechanisms.
Complivit® Calcium D3 does not affect the ability to drive vehicles or operate technically complex machinery.
Contraindications
Contraindications
Hypersensitivity to the components of the drug.
Hypercalcemia (increased concentration of calcium in the blood).
Hypercalciuria (increased concentration of calcium in the urine).
Nephrolithiasis.
Hypervitaminosis D.
Decalcifying tumors (myeloma, bone metastases, sarcoidosis).
Active tuberculosis.
Severe renal failure.
The drug in tablet form is not used in children under 3 years of age.
Complivit® Calcium D3 contains aspartame, which is converted to phenylalanine in the body. Therefore, the drug should not be taken by patients with phenylketonuria.
The drug contains lactose and sucrose; therefore it is not recommended for patients with hereditary lactose intolerance, fructose, glucose-galactose malabsorption, lactase or sucrose/isomaltase deficiency.
Side effects
Side effects
The frequency of side effects of the drug is rated as follows:
Very frequent: > 1/10
Frequent: >1/100, < 1/10
Infrequent: >1/1000, < 1/100
Rare: >1/10,000, <1/1000
Very rare: <1/10,000
Metabolic and nutritional disorders: infrequent – hypercalcemia, hypercalciuria.
Gastrointestinal tract disorders: rarely – constipation, flatulence, nausea, abdominal pain, diarrhea, dyspepsia.
Skin and subcutaneous tissue disorders: very rare – allergic reactions (itching, rash, urticaria).
Overdose
Overdose
Symptoms of overdose (hypercalcemia): thirst, polyuria, anorexia, nausea, vomiting, constipation, abdominal pain, dizziness, muscle weakness, increased fatigue, bone pain, mental disorders, headache, fainting states, coma, nephrocalcinosis, urolithiasis, in severe cases – cardiac arrhythmias.
In prolonged use of excessive doses (over 2500 mg of calcium) – kidney damage, calcinosis of soft tissues.
Laboratory findings in overdose: hypercalciuria, hypercalcemia (plasma calcium about 2.6 mmol).
If signs of overdose are found, discontinue calcium and vitamin D as well as thiazide diuretics and cardiac glycosides, and seek medical attention.
Treatment: gastric lavage, fluid replacement, use of loop diuretics (e.g., furosemide), glucocorticosteroid drugs, calcitonin, bisphosphonates, calcium-limiting diet, and hemodialysis.
Plasma electrolyte levels, renal function and urine output should be monitored.
In severe cases, central venous pressure (CVP) and electrocardiogram (ECG) monitoring are necessary.
Pregnancy use
Pregnancy use
Complivit® Calcium D3 is used during pregnancy to replenish calcium and vitamin D deficiency in the body. During pregnancy, the daily dose should not exceed 1500 mg of calcium and 600 ME of vitamin D3. Hypercalcemia in overdose during pregnancy may have adverse effects on the developing fetus.
Similarities
Similarities
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Temperature not exceeding 25 0C. Keep out of reach of children. |
| Manufacturer | Pharmstandard-UfaVITA, Russia |
| Medication form | chewable tablets |
| Brand | Pharmstandard-UfaVITA |
Other forms…
Related products
Buy Complivit Calcium D3, orange 120 pcs with delivery to USA, UK, Europe and over 120 other countries.