No products in the cart.
Complivit Calcium D3 for kids, 43 g
€8.99 €7.87
Out of stock
(E-mail when Stock is available)
Description
Combination drug designed to replenish calcium and vitamin D3 deficiency in the body.
Pharmacodynamics. Calcium is involved in the formation of bone tissue, increases its density, participates in the mineralization of teeth, in the regulation of nerve conduction and muscle contractions, in maintaining the stability of cardiac activity, in the process of blood clotting.
Vitamin D3 (colecalciferol) regulates calcium and phosphorus metabolism in the body, increases calcium absorption in the intestine, promotes bone mineralization, formation of bone skeleton and teeth in children. The drug reduces the production of parathyroid hormone, which is a stimulant of increased bone resorption.
Indications
Indications
Prevention of calcium and vitamin D3 deficiency in young children.
Pharmacological effect
Pharmacological effect
A combined drug intended to compensate for the deficiency of calcium and vitamin D3 in the body.
Pharmacodynamics. Calcium is involved in the formation of bone tissue, increases its density, participates in the mineralization of teeth, in the regulation of nerve conduction and muscle contractions, in maintaining the stability of cardiac activity, and in the process of blood clotting.
Vitamin D3 (colecalciferol) regulates the exchange of calcium and phosphorus in the body, increases the absorption of calcium in the intestines, promotes bone mineralization, the formation of the bone skeleton and teeth in children. The drug reduces the production of parathyroid hormone, which is a stimulator of increased bone resorption.
Special instructions
Special instructions
To avoid overdose, do not use Complivit calcium D3 simultaneously with vitamin complexes containing calcium and vitamin D3.
When using vitamin D3 prophylactically, it is necessary to keep in mind the possibility of overdose, especially in children (you should not prescribe more than 10-15 mg per year).
Long-term use in high doses leads to chronic hypervitaminosis D3. With long-term treatment, it is necessary to monitor the concentration of Ca2+ in the blood and urine (especially when combined with thiazide diuretics).
It should be borne in mind that sensitivity to vitamin D varies from patient to patient, and in some patients taking even therapeutic doses can cause symptoms of hypervitaminosis.
The sensitivity of newborns to vitamin D varies, some may be sensitive even to very low doses, so prophylaxis should be carried out under the supervision of a physician.
Breastfed newborns, especially those born to mothers with dark skin and/or insufficient sun exposure, are at high risk of developing vitamin D deficiency.
Active ingredient
Active ingredient
Calcium carbonate, Colecalciferol
Composition
Composition
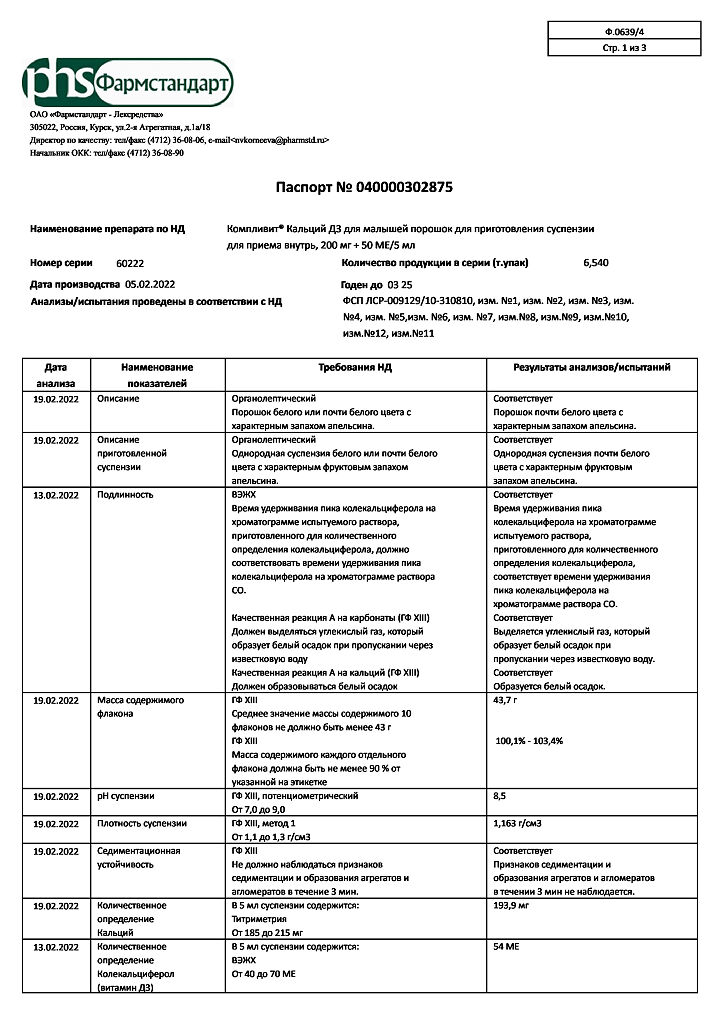
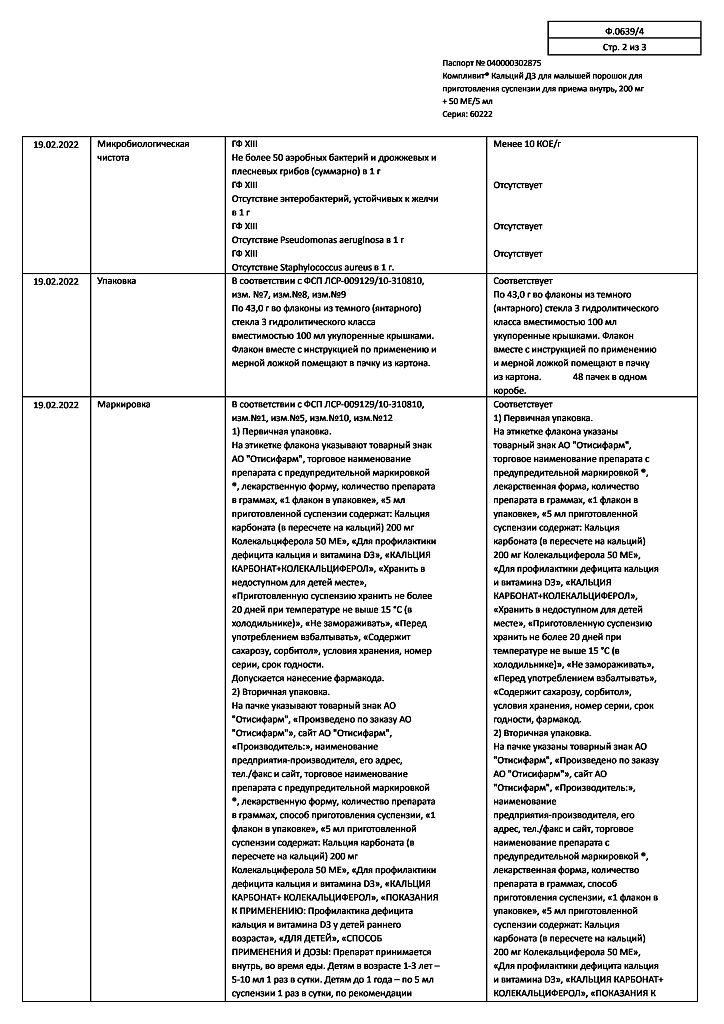
5 ml:
Active substances:
calcium carbonate (in terms of calcium) – 500 mg (200 mg);
colecalciferol (in terms of 100% colecalciferol) – 0.00135 mg (50 IU), in the form of granules containing: colecalciferol, D, L-alpha tocopherol, medium chain triglycerides, sucrose, acacia gum, corn starch, calcium phosphate, water.
Excipients:
sorbitol (sorbitol) 1.479 g,
pregelatinized starch (pregel) – 0.15 g,
colloidal silicon dioxide (aerosil) – 0.0108 g,
orange flavoring – 0.01 g.
Pregnancy
Pregnancy
During pregnancy and during breastfeeding, the use of the drug is possible on the recommendation of a doctor.
The daily dose should not exceed 1500 mg of calcium and 600 IU of vitamin D3.
Hypercalcemia due to overdose and excessive intake of vitamin D3 during pregnancy can have adverse effects on the developing fetus.
Calcium and vitamin D3
may pass into breast milk, so when the mother takes the drug during
breastfeeding, it is necessary to take into account the intake of calcium and vitamin D from
other sources.
Contraindications
Contraindications
Hypersensitivity, hypercalcemia, hypercalciuria, calcium nephrourolithiasis, hypervitaminosis D, decalcifying tumors (myeloma, bone metastases, sarcoidosis), osteoporosis due to immobilization, active form of pulmonary tuberculosis; sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption.
WITH CAUTION
Renal failure, benign granulomatosis, taking cardiac glycosides and thiazide diuretics, pregnancy, lactation.
Side Effects
Side Effects
Allergic reactions. When using the drug in recommended doses, no other side effects were identified. If recommended doses are exceeded or other drugs containing calcium are taken simultaneously, hypercalcemia and hypercalciuria (increased calcium levels in the blood and urine) may develop.
Possible side effects of vitamin D3 also include: decreased appetite, polyuria, constipation, headache, myalgia, arthralgia, increased blood pressure, arrhythmias, impaired renal function, exacerbation of the tuberculosis process in the lungs.
Possible side effects of calcium carbonate also include: gastralgia, constipation or diarrhea, flatulence, nausea, secondary increased gastric secretion.
Interaction
Interaction
Calcium and vitamin D3 preparations reduce the absorption of bisphosphonates, digoxin, iron supplements and tetracycline antibiotics (an interval between doses of at least 2-3 hours is required).
It is possible to enhance the effect of cardiac glycosides (with simultaneous use, monitoring of the ECG and the patient’s condition is necessary).
Phenytoin, barbiturates, and primidone reduce the effect of vitamin D3 by enhancing its metabolism.
Vitamin A, tocopherol, ascorbic acid, pantothenic acid, thiamine, riboflavin weaken the toxic effect of vitamin D3.
Glucocorticosteroids reduce the absorption of calcium ions in the intestine.
Cholestyramine, colestipol and mineral oils reduce the absorption of vitamin D3 and require an increase in its dosage.
Thiazide diuretics increase the risk of hypercalcemia.
Vitamin D increases the absorption of phosphorus-containing drugs and the risk of hyperphosphatemia. When used simultaneously with sodium fluoride, the interval between doses should be at least 2 hours; with oral forms of tetracyclines – at least 3 hours.
Long-term therapy with vitamin D3 against the background of simultaneous use of Al3+ and Mg2+-containing antacids increases their concentration in the blood and the risk of intoxication (especially in the presence of chronic renal failure).
Concomitant use with other vitamin D analogues and calcium preparations increases the risk of developing hypervitaminosis D.
Overdose
Overdose
Symptoms: thirst, polyuria, loss of appetite, nausea, vomiting, constipation, dizziness, weakness, headache, fainting, coma; with long-term use, calcification of blood vessels and tissues. Laboratory indicators in case of overdose: hypercalciuria, hypercalcemia.
Treatment: stopping the drug, seeing a doctor, calcium-restricted diet, rehydration, diuretics, glucocorticosteroids, and in severe cases, hemodialysis.
Storage conditions
Storage conditions
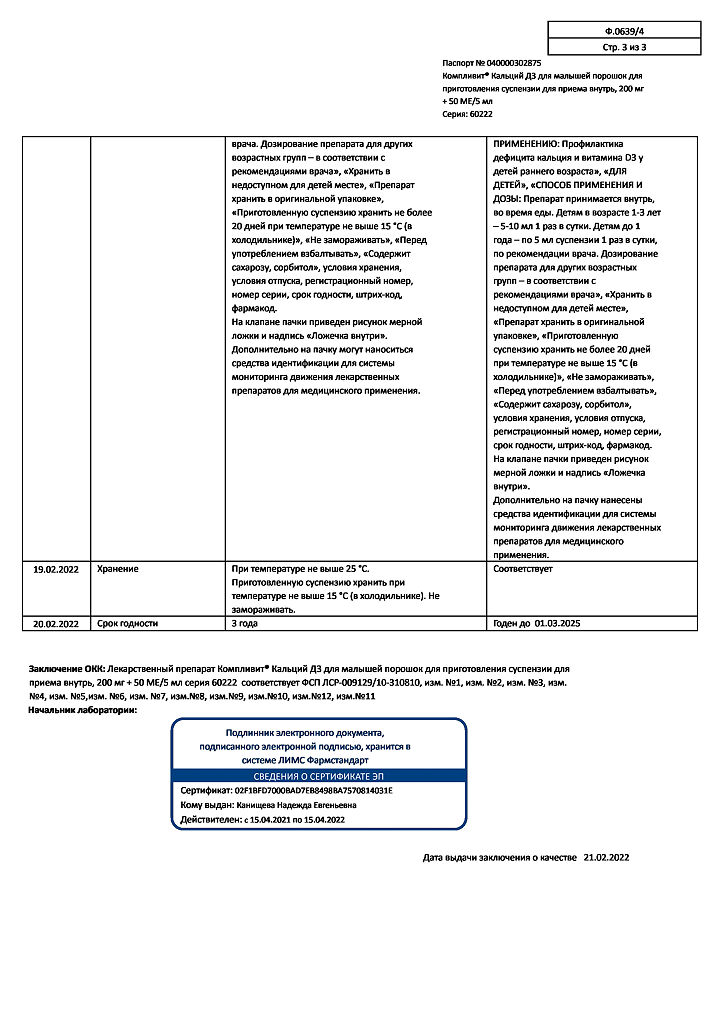
Store in a place protected from light, at a temperature not exceeding 25 °C.
Shelf life
Shelf life
3 years.
Store the prepared suspension for no more than 20 days.
Do not use
expiration date indicated on the packaging.
Manufacturer
Manufacturer
Pharmstandard-UfaVITA, Russia
Additional information
| Shelf life | 3 years. Store the prepared suspension for no more than 20 days. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | Store in a dark place at a temperature not exceeding 25 ° C. |
| Manufacturer | Pharmstandard-UfaVITA, Russia |
| Medication form | Powder for oral suspension |
| Brand | Pharmstandard-UfaVITA |
Related products
Buy Complivit Calcium D3 for kids, 43 g with delivery to USA, UK, Europe and over 120 other countries.