No products in the cart.
Coldact Flux Plus, capsules 10 pcs
€11.63 €10.75
Description
A combined drug of prolonged action.
Chlorphenamine has anti-allergic effect, eliminates lacrimation, itching of the eyes and nose.
Paracetamol has antipyretic and analgesic effect: it reduces pain syndrome caused by colds – sore throat, headache, muscle and joint pain, reduces fever.
Phenylephrine has a vasoconstrictor effect – reduces swelling and hyperemia of the mucous membranes of the upper airways and sinuses.
Indications
Indications
Symptomatic treatment of colds, flu, acute respiratory viral infections (fever, pain, rhinorrhea).
Pharmacological effect
Pharmacological effect
Combined drug with prolonged action.
Chlorphenamine has an antiallergic effect, eliminates lacrimation, itching in the eyes and nose.
Paracetamol has an antipyretic and analgesic effect: it reduces the pain syndrome observed during colds – sore throat, headache, muscle and joint pain, and reduces high temperature.
Phenylephrine has a vasoconstrictor effect – reduces swelling and hyperemia of the mucous membranes of the upper respiratory tract and paranasal sinuses.
Special instructions
Special instructions
You should not continue taking Coldact Flu Plus without consulting a doctor if the temperature persists for more than 3 days.
Without a doctor’s instructions, the drug should not be used by patients undergoing treatment with other medications, in particular MAO inhibitors. If, despite taking the drug, the disease is accompanied by ongoing fever or repeated increases in temperature are observed, you should consult a doctor. Do not take with alcohol or combine with other drugs containing paracetamol. When using Coldact Flu Plus, it is not advisable to use sleeping pills, tranquilizers and other psychotropic drugs.
Distorts laboratory test results in the quantitative determination of glucose and uric acid in plasma. In case of long-term treatment, peripheral blood parameters and the functional state of the liver are monitored.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
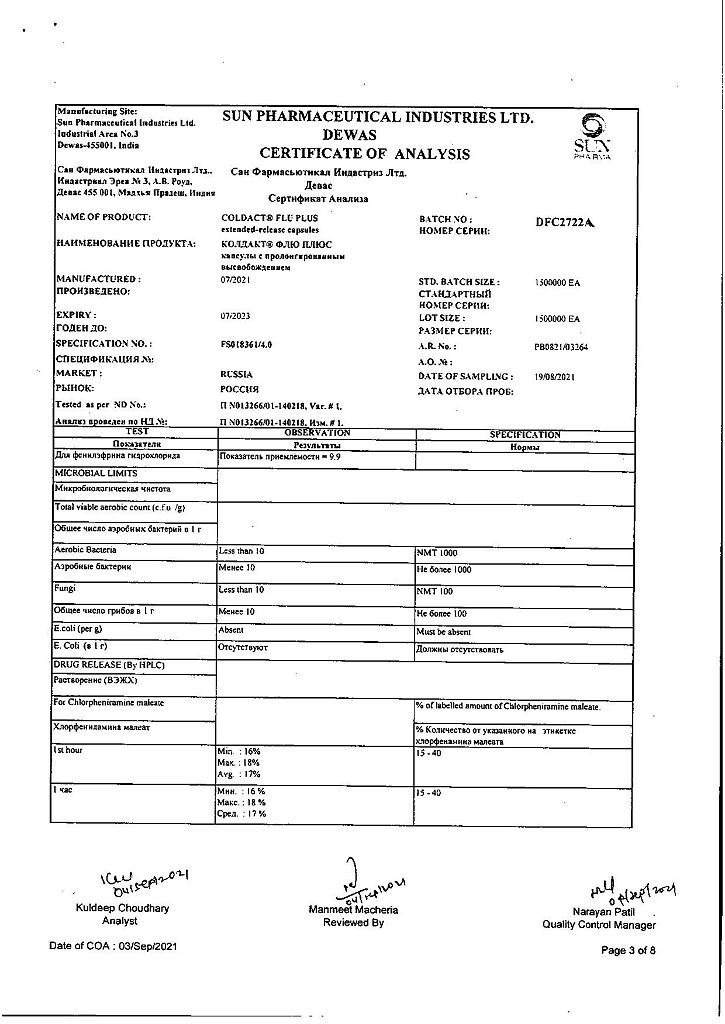
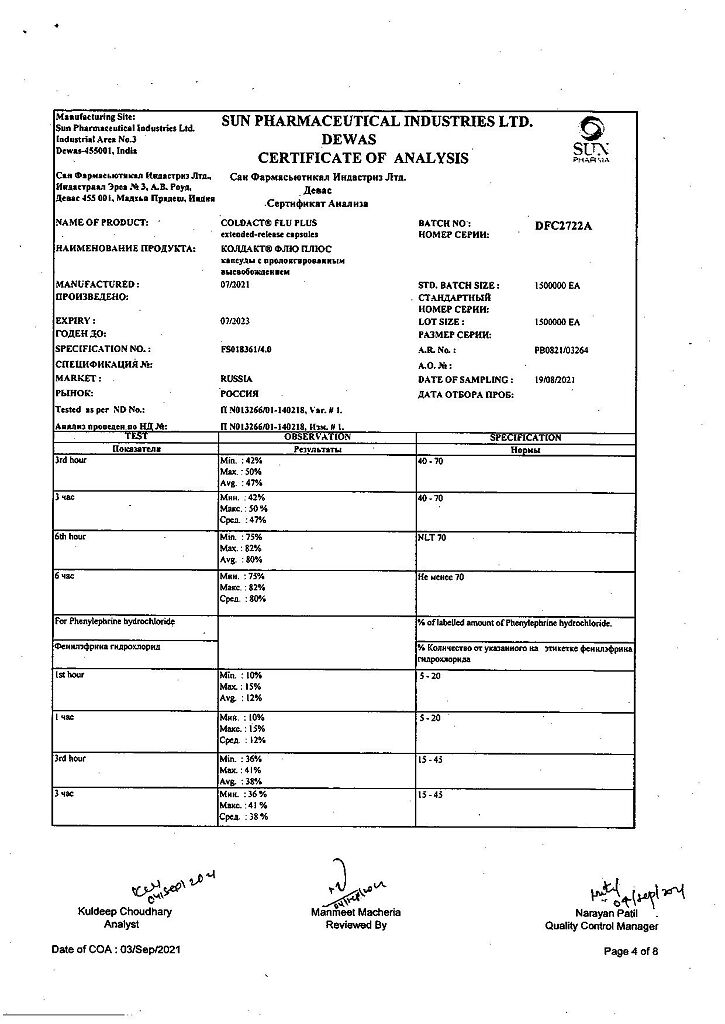
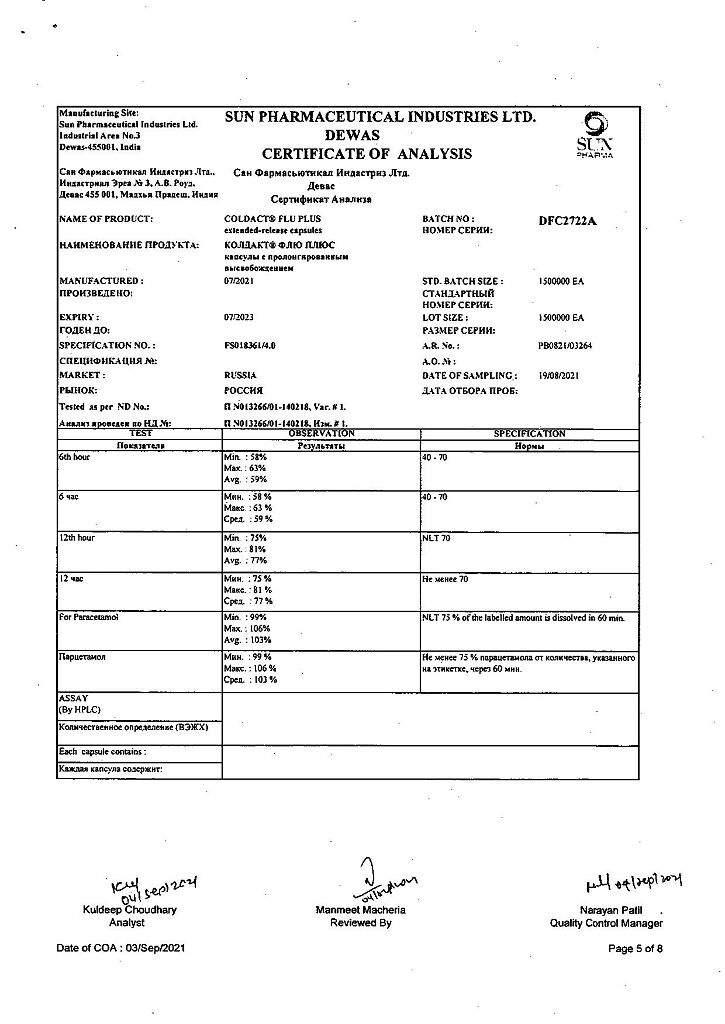
Paracetamol, Phenylephrine, Chlorphenamine
Composition
Composition
Active Ingredients:
Chlorphenamine maleate 8 mg
Paracetamol 200 mg
Phenylephrine hydrochloride 25 mg
Auxiliary ingredients:
talc 68.25 mg,
hypromellose 4.5 mg,
ethylcellulose 29 mg,
diethyl phthalate 5.8 mg,
microcrystalline cellulose 28.01 mg,
povidone – 6.41 mg,
purified water* q.s.,
isopropanol* q.s.,
sodium disulfite 0.16 mg,
disodium edetate 0.32 mg,
crimson dye (Ponceau 4R) 0.35 mg,
sunset yellow dye 0.01 mg,
dye quinoline yellow 0.08 mg,
granules Non Pareil Seeds 18/22 (sugar granules: sucrose, starch syrup) 124.11 mg.
Capsule shell:
gelatin q.s. up to 100%,
purified water 14-15%,
methyl parahydroxybenzoate 0.2%,
Azorubine dye 0.35%,
crimson dye (Ponceau 4R) 0.47%.
*lost during production
Pregnancy
Pregnancy
Coldact Flu Plus capsules are not recommended for use during pregnancy and lactation.
Use in children
Contraindicated for children under 12 years of age.
Contraindications
Contraindications
Hypersensitivity to any of the components included in the composition.
Severe atherosclerosis of the coronary arteries.
Arterial hypertension.
Diabetes mellitus.
Thyrotoxicosis.
Angle-closure glaucoma.
Severe diseases of the liver, kidneys, heart, bladder, peptic ulcer of the stomach and duodenum.
Diseases of the pancreas.
Difficulty urinating with prostate adenoma.
Diseases of the blood system.
Deficiency of the enzyme glucose-6-phosphate dehydrogenase.
Children’s age up to 12 years.
With caution: congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes), bronchial asthma and chronic obstructive pulmonary disease.
Side Effects
Side Effects
Increased blood pressure.
Tachycardia.
Drowsiness.
Sleep disturbance.
Dizziness.
Increased excitability.
Dry mucous membranes.
Mydriaz.
Paresis of accommodation.
Increased intraocular pressure.
Decreased appetite.
Nausea.
Epigastric pain.
Anemia.
Very rarely, urinary retention.
Allergic reactions (skin rash, itching, urticaria, angioedema)
Rarely
Anemia.
Thrombocytopenia.
Leukopenia.
Agranulocytosis.
With long-term use in high doses, hepatotoxic and nephrotoxic effects are possible.
Interaction
Interaction
Increased risk of hematotoxicity.
The risk of developing hematotoxic effects of paracetamol increases with the simultaneous administration of barbiturates, diphenine, carbamazepine, rifampicin, zidovudine and other inducers of microsomal liver enzymes. Enhances the effects of sedatives, ethanol, monoamine oxidase inhibitors.
Antidepressants, phenothiazine derivatives, antiparkinsonian and antipsychotic drugs
Antidepressants, phenothiazine derivatives, antiparkinsonian and antipsychotic drugs increase the risk of developing urinary retention, dry mouth, and constipation.
Glucocorticosteroids
Glucocorticosteroids increase the risk of developing glaucoma. Paracetamol reduces the effectiveness of uricosuric drugs. Chlorphenamine simultaneously with furazolidone can lead to a hypertensive crisis and hyperrexia.
Tricyclic antidepressants
Tricyclic antidepressants enhance the adrenomimetic effect of phenylephrine; simultaneous administration of halothane increases the risk of developing ventricular arrhythmia. Reduces the hypotensive effect of guanethidine, which in turn enhances the alpha-adrenergic stimulating activity of phenylephrine.
Overdose
Overdose
Symptoms (due to the presence of paracetamol in the composition) appear after taking more than 10-15 g: pallor of the skin, decreased appetite, nausea, vomiting, hepatonecrosis, increased activity of liver transaminases, increased prothrombin time.
Treatment: gastric lavage in the first 6 hours, administration of SH-group donors and precursors for the synthesis of glutathione-methionine 8-9 hours after overdose and N-acetylcysteine after 12 hours.
In case of accidental overdose, consult a doctor immediately, regardless of whether any symptoms of overdose are noted or not.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C, out of the reach of children.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Sun Pharmaceutical Industries Ltd, India
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | In a dry place protected from light at a temperature not exceeding 25 °C, out of the reach of children. |
| Manufacturer | Sun Pharmaceutical Industries Ltd, India |
| Medication form | slow-release capsules |
| Brand | Sun Pharmaceutical Industries Ltd |
Related products
Buy Coldact Flux Plus, capsules 10 pcs with delivery to USA, UK, Europe and over 120 other countries.