No products in the cart.
Description
Pharmacodynamics
Antitumor agent containing platinum. Mechanism of action is similar to that of alkylating drugs and consists in disruption of DNA strands function and formation of cross-links between them.
Pharmacokinetics
Cisplatin poorly penetrates through the BBB. It is rapidly metabolized by non-enzymatic conversion to inactive metabolites. Binding to proteins (as metabolites) is 90%.
T1/2 in the initial phase is 25-49 min; in the final phase with normal renal excretory function – 58-73 h, with anuria – up to 240 h. Excreted by the kidneys, 27-43% after 5 days; platinum can be detected in tissues for 4 months after administration.
Indications
Indications
Germ cell tumor of the testicle or ovaries;
ovarian cancer;
cancer of the uterus;
uterine sarcoma;
cancer of the cervix and fallopian tubes;
ovarian cancer;
cancer of the renal pelvis and ureters;
bladder and urethral cancer;
prostate and penile cancer;
osteogenic sarcoma;
Ewing’s sarcoma;
neuroblastoma;
retinoblastoma;
soft tissue sarcoma;
lymphoma;
uterine chorionic carcinoma;
medulloblastoma;
skin cancer;
melanoma;
tumor of the head and neck;
esophageal cancer;
lung cancer;
stomach cancer;
colon cancer;
malignant thymoma;
mesothelioma.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Antitumor agent, contains platinum. The mechanism of action is similar to the action of alkylating drugs and consists in disrupting the function of DNA strands and the formation of cross-links between them.
Pharmacokinetics
Cisplatin penetrates the BBB poorly. Rapidly metabolized by non-enzymatic conversion to inactive metabolites. Protein binding (in the form of metabolites) is 90%.
T1/2 in the initial phase is 25-49 minutes; in the final phase with normal renal excretory function – 58-73 hours, with anuria – up to 240 hours. Excreted by the kidneys, 27-43% after 5 days; platinum can be detected in tissues for 4 months after administration.
Special instructions
Special instructions
It is not recommended to use cisplatin in patients with chickenpox (including recent or after contact with sick people), herpes zoster and other acute infectious diseases.
Use with caution in patients with gout or nephrolithiasis (including a history), as well as in patients who have previously received cytotoxic chemotherapy or radiation therapy.
Before starting and during treatment with cisplatin, it is necessary to monitor the peripheral blood picture, laboratory data of kidney and liver function, indicators of water-electrolyte metabolism and uric acid levels, conduct audiometry and neurological examinations. The first manifestations of the nephrotoxic effect of cisplatin occur in the 2nd week after administration and are manifested by increased levels of creatinine, uric acid, residual nitrogen and/or decreased creatinine clearance. To reduce nephrotoxicity, before starting treatment, it is recommended to administer an intravenous infusion of 0.9% sodium chloride solution or 5% glucose solution and additionally prescribe mannitol.
Vaccination of patients and their family members is not recommended during cisplatin therapy.
Experimental studies have established the carcinogenic and mutagenic effects of cisplatin.
Active ingredient
Active ingredient
Cisplatin
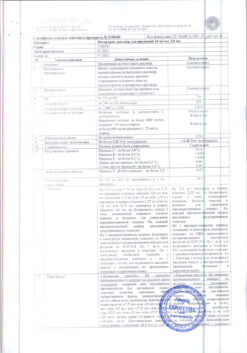
Composition
Composition
1 ml of concentrate contains:
Active ingredients:
cisplatin 500 mcg.
There are 10, 50 or 100 ml of concentrate in a light-protective glass bottle.
There are 1, 4, 6, 10, 25 or 40 bottles in a cardboard package.
Pregnancy
Pregnancy
Cisplatin is contraindicated during pregnancy. If it is necessary to use it during lactation, the issue of stopping breastfeeding should be decided.
Women of childbearing potential should use reliable methods of contraception during cisplatin therapy.
Experimental studies have established the teratogenic and embryotoxic effects of cisplatin.
Contraindications
Contraindications
Severe renal dysfunction;
hearing impairment;
polyneuritis;
inhibition of hematopoiesis;
pregnancy;
hypersensitivity to cisplatin.
Side Effects
Side Effects
From the digestive system: nausea, vomiting, stomatitis, anorexia.
From the hematopoietic system: leukopenia, anemia, thrombocytopenia.
From the central nervous system: convulsions, peripheral neuropathy, optic neuritis, color vision disorders, ototoxicity.
Metabolism: hyperuricemia, hypocalcemia, hypomagnesemia, syndrome of inappropriate ADH secretion.
From the reproductive system: amenorrhea, azoospermia.
From the cardiovascular system: tachycardia, arterial hypotension.
Allergic reactions: skin rash, angioedema, hoarseness.
Other: nephrotoxic effect.
Interaction
Interaction
Concomitant use of cisplatin with uricosuric anti-gout drugs may increase the risk of nephropathy.
Combined use with antihistamines, phenothiazines, and thioxanthenes may mask the symptoms of the ototoxic effect of cisplatin.
When used simultaneously with drugs that have ototoxic, nephrotoxic, neurotoxic effects, toxic effects may be enhanced.
Overdose
Overdose
The main expected complications of overdose are impaired renal function, liver dysfunction, visual impairment (including retinal detachment) and hearing impairment (deafness), severe myelosuppression, uncontrollable nausea and vomiting and/or neuritis. Overdose can result in death.
There is no known antidote for Cisplatin overdose. The effect, at least partially, is achieved only with hemodialysis if it is used within the first three hours after an overdose, since platinum quickly binds to plasma proteins. To eliminate the symptoms of overdose, symptomatic therapy is used.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 15–25 °C (do not freeze)
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Lance Farm, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In the dark place at 15-25 °C (do not freeze) |
| Manufacturer | Lance Farm, Russia |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Lance Farm |
Related products
Buy Cisplatin-LNS concentrate 0.5 mg/ml, 20 ml with delivery to USA, UK, Europe and over 120 other countries.