No products in the cart.
Cifran ST, 500 mg+600 mg 10 pcs
€13.44 €11.76
Description
Pharmacological action
Pharmacological action – broad spectrum antibacterial, bactericidal.
Indications
Indications
Treatment of mixed infections caused by sensitive anaerobic and aerobic microorganisms:
chronic sinusitis;
lung abscess;
empyema;
intra-abdominal infections;
inflammatory gynecological diseases;
postoperative infections (with the possible presence of aerobic and anaerobic bacteria);
chronic osteomyelitis;
skin and soft tissue infections;
diabetic foot ulcers;
bedsores;
Oral infections (including periodontitis and periostitis).
Treatment of diarrhea or dysentery of amoebic or mixed (amebic and bacterial) etiology.
Pharmacological effect
Pharmacological effect
Pharmacological action
Pharmacological action – broad-spectrum antibacterial, bactericidal.
Special instructions
Special instructions
It is recommended to avoid excessive exposure to sunlight during the course of therapy with Cifran® ST, since phototoxicity reactions have been observed in some patients receiving fluoroquinolones. If phototoxicity reactions occur, you should immediately stop using the drug.
When using tinidazole, it is possible (but rare) to develop generalized urticaria, swelling of the face and larynx, decreased blood pressure, bronchospasm and dyspnea. If the patient is allergic to any imidazole derivative, cross-sensitivity to tinidazole may also develop; the development of a cross-allergic reaction to ciprofloxacin is also possible in patients with allergies to other fluoroquinolone derivatives. Therefore, if the patient has had any allergic reactions to similar drugs, the possibility of cross-allergic reactions to Cifran® ST should be taken into account.
When tinidazole is used together with alcohol, painful abdominal cramps, nausea and vomiting may occur. Therefore, the combined use of Tsifran® ST and alcohol is contraindicated.
To avoid the development of crystalluria, it is unacceptable to exceed the recommended daily dose; it is also necessary to have sufficient fluid intake and maintain an acidic urine reaction. Causes dark coloration of urine.
During treatment, you should refrain from engaging in potentially hazardous activities that require increased attention and speed of mental and motor reactions.
For patients with epilepsy, a history of seizures, vascular diseases and organic brain damage, due to the risk of developing adverse reactions from the central nervous system, the drug should be prescribed only for health reasons.
If severe and prolonged diarrhea occurs during or after treatment, the diagnosis of pseudomembranous colitis should be excluded, which requires immediate discontinuation of the drug and the appointment of appropriate treatment.
If pain appears in the tendons or when the first signs of tenosynovitis appear, treatment should be stopped.
During treatment, peripheral blood patterns should be monitored.
The safety and effectiveness of use for the treatment and prevention of anaerobic infections in children under 12 years of age has not been established.
Active ingredient
Active ingredient
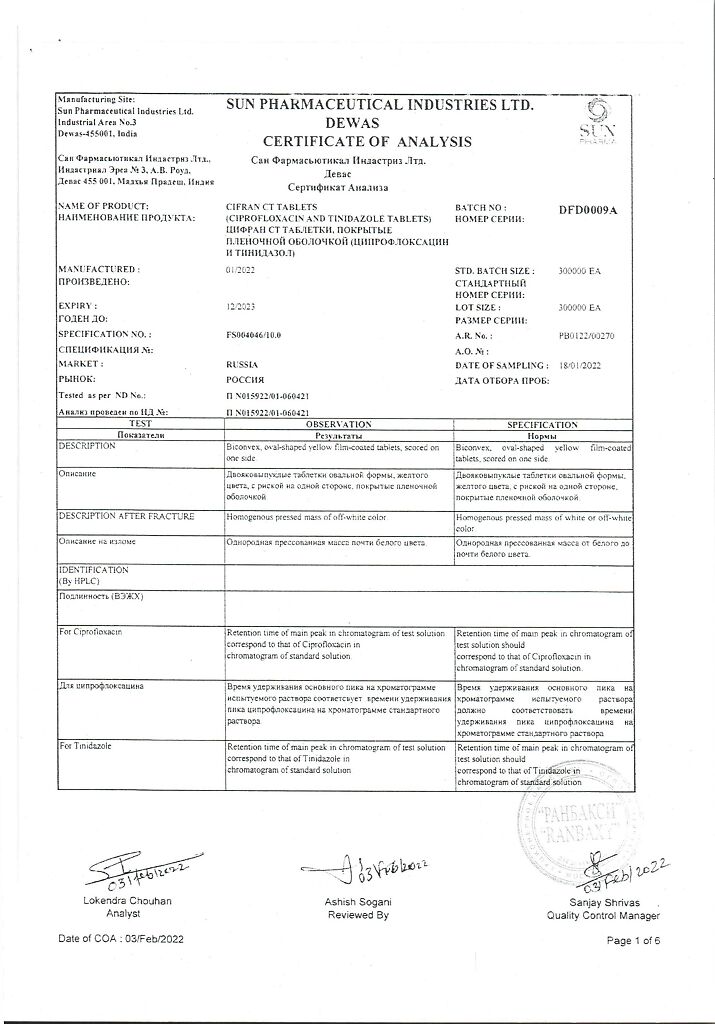
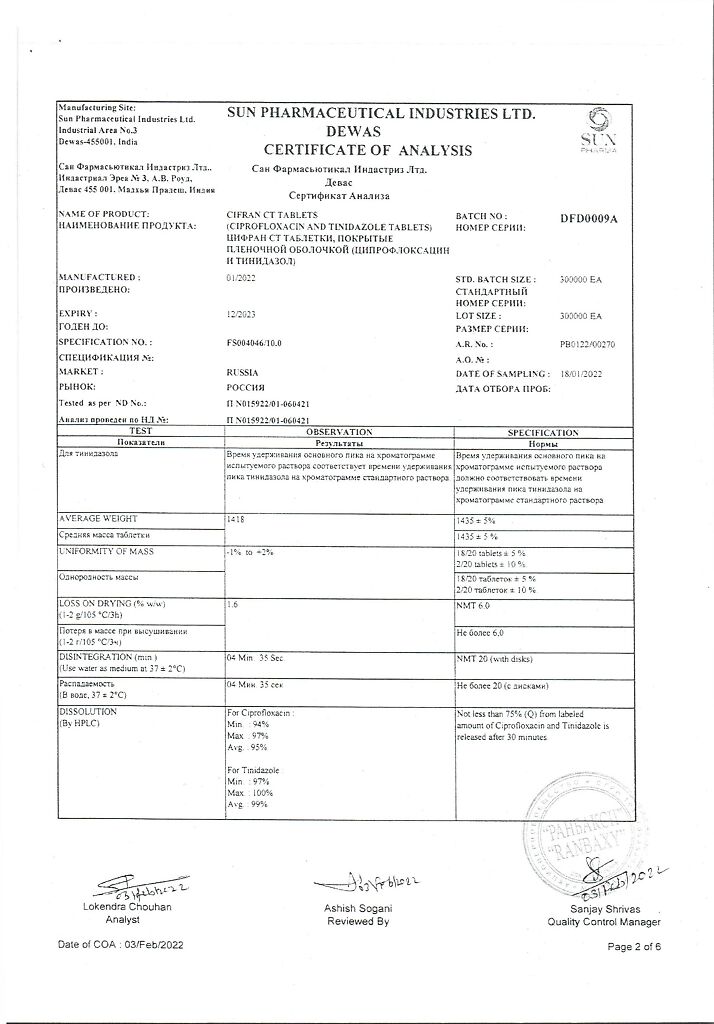
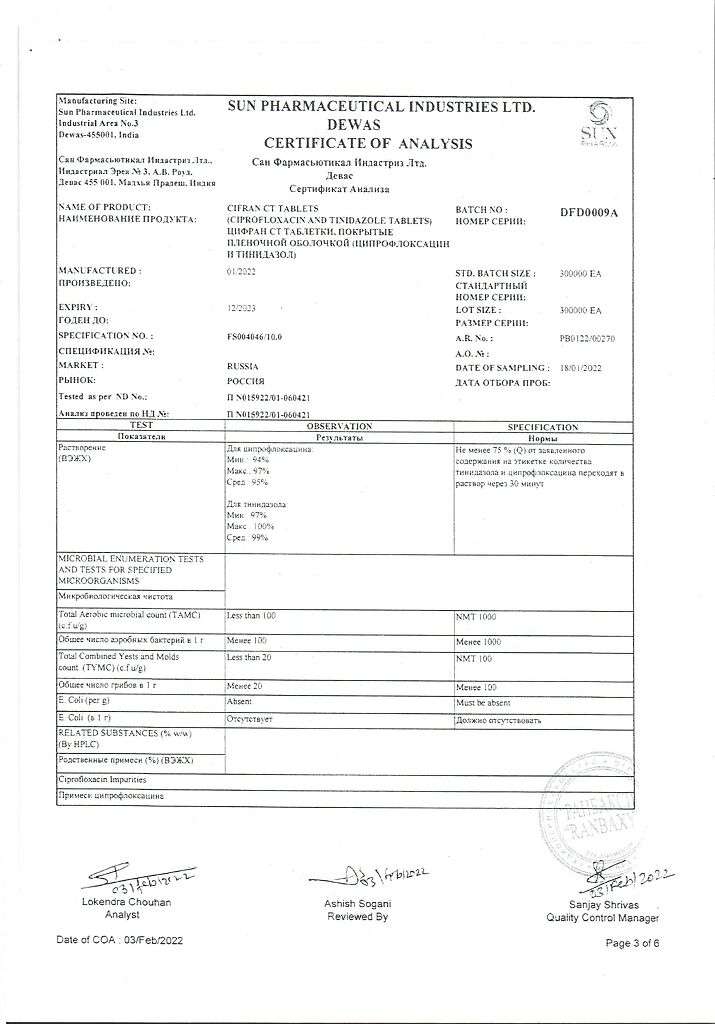
Tinidazole, Ciprofloxacin
Composition
Composition
1 film-coated tablet contains:
Active ingredients:
ciprofloxacin hydrochloride USP equivalent to ciprofloxacin – 500.00 mg
tinidazole VR – 600 mg,
Excipients: microcrystalline cellulose, colloidal anhydrous silicon, magnesium stearate, sodium starch glycolate, sodium lauryl sulfate.
Ingredients of the outer layer of granules: sodium starch glycolate, purified talc, sodium lauryl sulfate, microcrystalline cellulose, colloidal anhydrous silicon, magnesium stearate.
Shell: yellow opadry, purified water.
Pregnancy
Pregnancy
Use during pregnancy is not recommended. Tinidazole may be carcinogenic and mutagenic. Ciprofloxacin penetrates the hematoplacental barrier.
Use is contraindicated during breastfeeding. Tinidazole and ciprofloxacin are excreted into breast milk. Therefore, during lactation, if the use of the drug is necessary, breastfeeding should be stopped.
Contraindications
Contraindications
Cifran ST is contraindicated for use in patients with hypersensitivity (allergy) to any fluoroquinolone or imidazole derivatives.
Tsifran ST is also contraindicated in patients with a history of hematological diseases. With suppression of bone marrow hematopoiesis, acute porphyria.
Tsifran ST is contraindicated in patients with organic neurological lesions.
Tsifran ST is contraindicated in pediatric patients (under 18 years of age).
With caution
The drug should be used with caution in patients with severe cerebral atherosclerosis, cerebrovascular accidents, mental illness, epilepsy, epileptic syndrome, severe renal and/or liver failure.
When using the drug in the elderly, no significant problems were noted. However, in elderly patients, an age-dependent decrease in renal function is possible, so caution should be exercised when using the drug in such patients.
Side Effects
Side Effects
From the digestive system: loss of appetite, dryness of the oral mucosa, “metallic” taste in the mouth, nausea, vomiting, diarrhea, abdominal pain, flatulence, cholestatic jaundice (especially in patients with previous liver diseases), hepatitis, hepatonecrosis.
From the side of the central nervous system: headache, dizziness, increased fatigue, impaired coordination of movements (including locomotor ataxia), dysarthria, peripheral neuropathy, rarely convulsions, weakness, tremor, insomnia, increased sweating, increased intracranial pressure, confusion, depression, hallucinations, as well as other manifestations of psychotic reactions, migraine, fainting, thrombosis cerebral arteries.
From the senses: disturbances of taste and smell, visual impairment (diplopia, changes in color vision), tinnitus, hearing loss.
From the cardiovascular system: tachycardia, heart rhythm disturbances, decreased blood pressure.
From the hematopoietic system: leukopenia, granulocytopenia, anemia, thrombocytopenia, leukocytosis, thrombocytosis, hemolytic anemia.
Laboratory indicators: hypoprothrombinemia, increased activity of liver transaminases and alkaline phosphatase, hypercreatinemia, hyperbilirubinemia, hyperglycemia.
From the urinary system: hematuria, crystalluria (with alkaline urine and low diuresis), glomerulonephritis, dysuria, polyuria, urinary retention, decreased nitrogen excretory function of the kidneys, interstitial nephritis.
Allergic reactions: skin itching, urticaria, formation of blisters accompanied by bleeding and the appearance of small nodules that form scabs, drug fever, pinpoint hemorrhages on the skin (petechiae), swelling of the face or larynx, shortness of breath, eosinophilia, increased photosensitivity, vasculitis, erythema nodosum, erythema multiforme (including syndrome Stevens-Johnson), toxic epidermal necrolysis (Lyell’s syndrome).
Other: arthralgia, arthritis, tenosynovitis, tendon ruptures, asthenia, myalgia, superinfections (candidiasis, pseudomembranous colitis), flushing.
Interaction
Interaction
Tinidazole
Enhances the effect of indirect anticoagulants (to reduce the risk of bleeding, the dose is reduced by 50%) and the effect of ethanol (disulfiram-like reactions).
Compatible with sulfonamides and antibiotics (aminoglycosides, erythromycin, rifampicin, cephalosporins).
It is not recommended to administer with ethionamide.
Phenobarbital speeds up metabolism.
Ciprofloxacin
Due to a decrease in the activity of microsomal oxidation processes in hepatocytes, it increases the concentration and lengthens T1/2 of theophylline (and other xanthines, such as caffeine), oral hypoglycemic drugs, indirect anticoagulants, and helps reduce the prothrombin index.
When combined with other antimicrobial drugs (beta-lactam antibiotics, aminoglycosides, clindamycin, metronidazole), synergism is usually observed.
Strengthens the nephrotoxic effect of cyclosporine, there is an increase in serum creatinine; in such patients it is necessary to monitor this indicator 2 times a week.
When taken simultaneously, it enhances the effect of indirect anticoagulants.
Oral administration together with iron-containing drugs, sucralfate and antacid drugs containing Mg2+, Ca2+, A13+ leads to a decrease in the absorption of ciprofloxacin, so it should be prescribed 1-2 hours before or 4 hours after taking the above drugs.
NSAIDs (excluding acetylsalicylic acid) increase the risk of seizures.
Didanosine reduces the absorption of ciprofloxacin due to the formation of complexes with Mg2+ and A13+ contained in didanosine.
Metoclopramide accelerates absorption, which leads to a decrease in the time to reach its maximum concentration.
Co-administration of uricosuric drugs leads to a slower elimination (up to 50%) and an increase in plasma concentrations of ciprofloxacin.
Overdose
Overdose
Treatment: therapy for an overdose of Cifran® ST should be symptomatic and include the following measures:
– induction of vomiting or gastric lavage,
– taking measures for adequate hydration of the body (infusion therapy);
– maintenance therapy.
There is no specific antidote.
Storage conditions
Storage conditions
In a dry place, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Sun Pharmaceutical Industries Ltd, India
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry place, at a temperature not exceeding 25 °C |
| Manufacturer | Sun Pharmaceutical Industries Ltd, India |
| Medication form | pills |
| Brand | Sun Pharmaceutical Industries Ltd |
Related products
Buy Cifran ST, 500 mg+600 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.