No products in the cart.
Cifran OD, 1000 mg 10 pcs
€9.02 €7.89
Out of stock
(E-mail when Stock is available)
Description
Intestinal infections, Cholecystitis, Skin infections, Infectious diseases, Osteomyelitis, Prostatitis, Urinary tract infections, Sinusitis, Respiratory tract infections, Lung inflammation (pneumonia)
According to rxlist.com (2020), oral ciprofloxacin is indicated to treat infections caused by sensitive strains of microorganisms.
Adult patients
. Skin and soft tissue infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Proteus vulgaris, Providencia stuartii, Morganella morganii, Citrobacter freundii, Pseudomonas aeruginosa, methicillin-sensitive strains of Staphylococcus aureus, methicillin-sensitive strains of Staphylococcus epidermidis or Streptococcus pyogenes.
Bone and joint infections caused by Enterobacter cloacae, Serratia marcescens, Pseudomonas aeruginosa.
Complicated intra-abdominal infections (in combination with metronidazole) caused by Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae or Bacteroides fragilis.
Infectious diarrhea caused by Escherichia coli (enterotoxigenic isolates), Campylobacter jejuni, Shigella boydii*, Shigella dysenteriae, Shigella flexneri, Shigella sonnei* when antibiotic therapy is indicated.
* Although treatment of infections caused by this microorganism in this organ system has demonstrated clinically significant results, efficacy has been studied in fewer than 10 patients.
Typhoid fever caused by Salmonella typhi. The efficacy of ciprofloxacin for eradication of the pathogen in chronic carriage has not been demonstrated.
Uncomplicated gonorrhea (cervical and urethral) caused by Neisseria gonorrhoeae.
Chronic bacterial prostatitis caused by Escherichia coli or Proteus mirabilis.
Infections of the lower respiratory tract
– Infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae or Streptococcus pneumoniae.
– Ciprofloxacin is not the drug of first choice in the treatment of suspected or confirmed pneumonia caused by Streptococcus pneumoniae.
– Treatment of exacerbations of chronic bronchitis caused by Moraxella catarrhalis.
Because the use of fluoroquinolones, including ciprofloxacin, is associated with a risk of serious adverse reactions (see Precautions), and in some patients exacerbations of chronic bronchitis are self-resolving, use for this indication is possible only if other treatments have been unsuccessful.
Urinary tract infections
– Urinary tract infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, Proteus mirabilis, Providencia rettgeri, Morganella morganii, Citrobacter koseri, Citrobacter freundii, Pseudomonas aeroportixermoctiae. Staphylococcus saprophyticus or Enterococcus faecalis, in adults.
– Acute uncomplicated cystitis caused by Escherichia coli or Staphylococcus saprophyticus, in adult women.
Because the use of fluoroquinolones, including ciprofloxacin, is associated with a risk of serious adverse reactions (see Precautions), and acute uncomplicated cystitis is a self-resolving condition in some patients, use for this indication is possible only if other treatments have been unsuccessful.
Pediatric patients
Complicated urinary tract infections and Escherichia coli-induced pyelonephritis in pediatric patients aged 1 to 17 years.
Despite efficacy in clinical trials, ciprofloxacin is not the drug of first choice in pediatric patients because of increased rates of adverse reactions compared to controls, including joint and/or surrounding tissue reactions. Ciprofloxacin use, like other fluoroquinolones, is associated with arthropathy and histopathological changes in load-bearing joints in young animals (see Pharmacology and Toxicology in Animals).
Acute sinusitis caused by Haemophilus influenzae, Streptococcus pneumoniae or Moraxella catarrhalis.
Because the use of fluoroquinolones, including ciprofloxacin, is associated with a risk of serious adverse reactions (see Precautions), and acute sinusitis is a self-resolving condition in some patients, use for this indication is possible only if other treatments have been unsuccessful.
Adult and pediatric patients
Pulmonary anthrax (after exposure). Ciprofloxacin is indicated in adult and pediatric patients ages birth to 17 years for inhalational anthrax (after exposure) to reduce the incidence or progression of disease after exposure to Bacillus anthracis in aerosol form.
The ciprofloxacin concentrations achieved in human serum served as a surrogate endpoint reasonably predictive of clinical efficacy and served as the initial basis for approval of this indication.1 Confirmatory clinical information on ciprofloxacin for post-exposure anthrax prophylaxis was obtained during the October 2001 anthrax bioterror attacks (see Clinical Studies).
Plague, including pneumonic and septic plague caused by Yersinia pestis, and for the prevention of plague in adult and pediatric patients from birth to 17 years. A study of the efficacy of ciprofloxacin could not be conducted in plague patients. This indication is based on efficacy studies performed only on animals (see Clinical Studies).
Indications
Indications
According to rxlist.com (2020), oral ciprofloxacin is indicated for the treatment of infections caused by susceptible strains of microorganisms.
Adult patients
Skin and soft tissue infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Proteus vulgaris, Providencia stuartii, Morganella morganii, Citrobacter freundii, Pseudomonas aeruginosa, methicillin-sensitive strains of Staphylococcus aureus, methicillin-sensitive strains of Staphylococcus epidermidis or Streptococcus pyogenes.
Bone and joint infections caused by Enterobacter cloacae, Serratia marcescens, Pseudomonas aeruginosa.
Complicated intra-abdominal infections (in combination with metronidazole) caused by Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae or Bacteroides fragilis.
Infectious diarrhea caused by Escherichia coli (enterotoxigenic isolates), Campylobacter jejuni, Shigella boydii*, Shigella dysenteriae, Shigella flexneri, Shigella sonnei*, when antibacterial therapy is indicated.
* Although treatment of infections caused by this organism in this organ system has demonstrated clinically significant results, efficacy has been studied in fewer than 10 patients.
Typhoid fever caused by Salmonella typhi. The effectiveness of ciprofloxacin for eradication of the pathogen in chronic carriage has not been demonstrated.
Uncomplicated gonorrhea (cervical and urethral) caused by Neisseria gonorrhoeae.
Chronic bacterial prostatitis caused by Escherichia coli or Proteus mirabilis.
Lower respiratory tract infections
– Infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae or Streptococcus pneumoniae.
– Ciprofloxacin is not the first choice drug for the treatment of suspected or confirmed pneumonia caused by Streptococcus pneumoniae.
– Treatment of exacerbations of chronic bronchitis caused by Moraxella catarrhalis.
Because the use of fluoroquinolones, including ciprofloxacin, is associated with a risk of serious adverse reactions (see “Precautions”), and in some patients exacerbation of chronic bronchitis is self-limiting, use for this indication is only possible if other treatments have failed.
Urinary tract infections
– Urinary tract infections caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Serratia marcescens, Proteus mirabilis, Providencia rettgeri, Morganella morganii, Citrobacter koseri, Citrobacter freundii, Pseudomonas aeroportixermoctiae. Staphylococcus saprophyticus or Enterococcus faecalis, in adults.
– Acute uncomplicated cystitis caused by Escherichia coli or Staphylococcus saprophyticus in adult women.
Because fluoroquinolones, including ciprofloxacin, are associated with a risk of serious adverse reactions (see Precautions) and acute uncomplicated cystitis is self-limiting in some patients, use for this indication should only be considered if other treatments have failed.
Pediatric patients
Complicated urinary tract infections and pyelonephritis due to Escherichia coli in pediatric patients aged 1 to 17 years.
Although effective in clinical trials, ciprofloxacin is not the first choice drug in pediatric patients due to an increased incidence of adverse reactions compared with controls, including reactions related to joints and/or surrounding tissue. Ciprofloxacin, like other fluoroquinolones, is associated with arthropathy and histopathological changes in weight-bearing joints in young animals (see Pharmacology and Toxicology in Animals).
Acute sinusitis caused by Haemophilus influenzae, Streptococcus pneumoniae or Moraxella catarrhalis.
Because fluoroquinolones, including ciprofloxacin, are associated with a risk of serious adverse reactions (see Precautions) and acute sinusitis is self-limiting in some patients, use for this indication should only be considered if other treatments have failed.
Adult and pediatric patients
Pulmonary anthrax (after exposure). Ciprofloxacin is indicated in adult and pediatric patients aged birth to 17 years with pulmonary anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis.
Ciprofloxacin concentrations achieved in human serum served as a surrogate endpoint reasonably predictive of clinical efficacy and provided the initial basis for approval of this indication1. Supporting clinical information for ciprofloxacin for anthrax post-exposure prophylaxis was obtained during the October 2001 anthrax bioterrorism attacks (see Clinical Studies).
Plague, including pneumonic and septicemic plague caused by Yersinia pestis, and for the prevention of plague in adult and pediatric patients from birth to 17 years of age. The effectiveness of ciprofloxacin could not be studied in patients with plague. This indication is based on an efficacy study conducted in animals only (see Clinical Studies).
Pharmacological effect
Pharmacological effect
Pharmacological group
Quinolones/fluoroquinolones
Pharmacology
Special instructions
Special instructions
Photosensitivity reactions have been observed in some patients receiving fluoroquinolones. Excessive exposure to direct sunlight and UV radiation should be avoided. If a photosensitivity reaction occurs, it is recommended to stop using the drug. Since Cifran® OD is a drug with possible reversible toxic effects on the kidneys, it is not recommended for use in patients with impaired renal function, with creatinine clearance <29 ml/min, or in patients on hemodialysis or peritoneal dialysis.
When using almost all antimicrobial drugs, including Cifran® OD, the development of pseudomembranous colitis is possible, varying in severity from mild to life-threatening. If severe and prolonged diarrhea occurs during or after treatment, the diagnosis of pseudomembranous colitis should be excluded, which requires immediate discontinuation of the drug and the appointment of appropriate treatment.
To avoid the development of crystalluria, it is unacceptable to exceed the recommended daily dose; it is also necessary to have sufficient fluid intake and maintain an acidic urine reaction.
For patients with epilepsy, a history of seizures, vascular diseases and organic brain lesions, due to the threat of adverse reactions from the central nervous system, Cifran® OD should be prescribed only for “life-saving” indications.
If pain appears in the tendons or when the first signs of tendovarinitis appear, treatment should be stopped (isolated cases of inflammation and even tendon rupture during treatment with fluoroquinolones have been described).
During treatment, you should refrain from engaging in potentially hazardous activities that require increased attention and speed of mental and motor reactions.
Active ingredient
Active ingredient
Ciprofloxacin
Composition
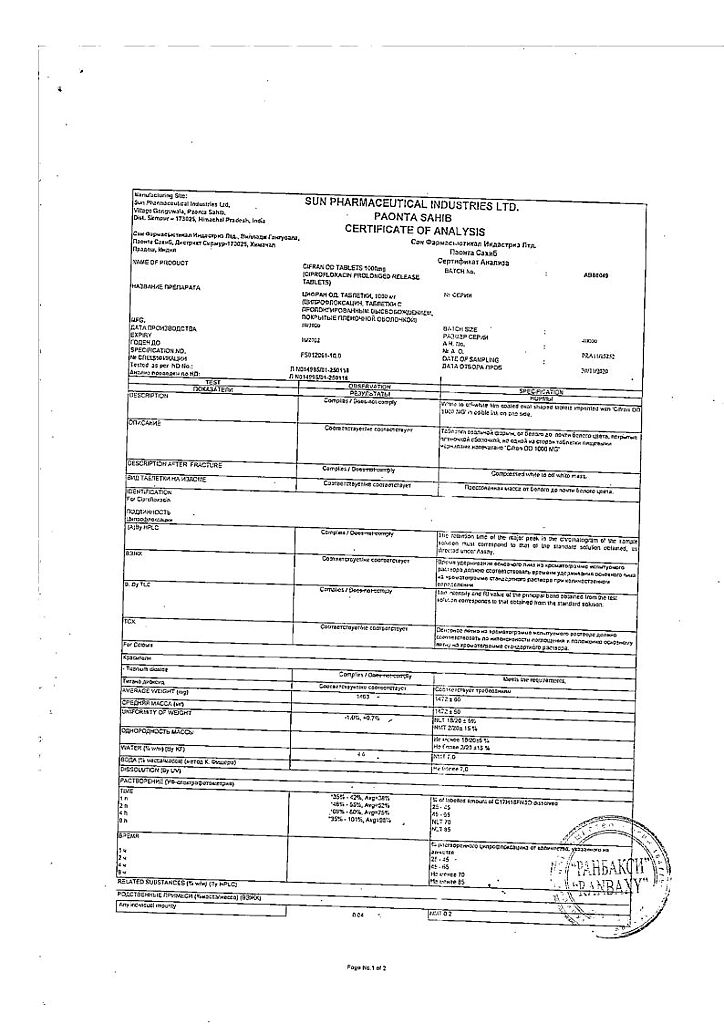
Composition
Active substance:
ciprofloxacin – 1000 mg.
Excipients:
sodium alginate (Keltone LVCR),
hypromellose,
sodium bicarbonate,
crospovidone (CLM),
magnesium stearate,
colloidal silicon dioxide (200),
talc.
Film casing:
opadry white 31B58910*, talc, hypromellose, purified water, black ink for writing (Opacode-S-l-17823 black).
Pregnancy
Pregnancy
Pregnancy
Summary of risks. Long-term experience with ciprofloxacin in pregnant women (several decades), based on available published information from case reports, case-control studies, and observational studies, suggests that there is no drug-associated risk of serious birth defects, miscarriage, or adverse maternal or fetal outcomes.
Available studies cannot definitively establish the absence of risk, because have methodological limitations, including small sample size, and some are not specific to ciprofloxacin.
Oral administration of ciprofloxacin in doses up to 100 mg/kg to pregnant female mice and rats during organogenesis and in doses up to 30 mg/kg in pregnant rabbits did not cause fetal malformations. These doses exceeded the MRDC of 0.3; 0.6 and 0.4 times in mice, rats and rabbits, respectively, based on body surface area.
The estimated background risk of serious birth defects and miscarriage in women is unknown. With any pregnancy, there is an underlying risk of developing a birth defect, miscarriage, or other adverse outcomes. In the general population in the United States, the estimated background risk of major congenital malformations and miscarriage in clinically diagnosed pregnancies is 2–4% and 15–20%, respectively.
Breast-feeding
Summary of risks. Published literature reports that ciprofloxacin is present in breast milk after IV and oral administration. There is no information on the effect of ciprofloxacin on milk production or on the nursing infant. Due to the potential risk of serious adverse reactions in breastfed infants, including arthropathy, as demonstrated in studies in young animals, breastfeeding is not recommended during treatment with ciprofloxacin for most indications during treatment and for an additional 2 days (5 T1/2 periods) after the last dose.
However, for pulmonary anthrax (post-exposure), an assessment of the risks and benefits of continuing breastfeeding while the mother (and possibly the baby) is receiving ciprofloxacin is necessary. The developmental and health benefits of breastfeeding, the clinical need for maternal use of ciprofloxacin, and the potential for side effects in the breastfed infant should be considered.
Clinical warnings
Ciprofloxacin may cause changes in intestinal flora in a nursing infant. It is recommended to monitor the condition of a breastfed baby for the appearance of loose or bloody stools and candidiasis (thrush, diaper rash).
Contraindications
Contraindications
Hypersensitivity to ciprofloxacin and other quinolones; simultaneous use with tizanidine.
Side Effects
Side Effects
The following serious and important adverse reactions are described in detail in the Precautions section:
– loss of ability to work and potentially irreversible serious adverse reactions (see “Precautions”);
– tendonitis and tendon rupture (see “Precautions”);
– peripheral neuropathy (see “Precautions”);
– effect on the central nervous system (see “Precautions”);
– exacerbation of myasthenia gravis (see “Precautions”);
– other serious and sometimes fatal reactions (see “Precautions”);
– hypersensitivity reactions (see “Precautions”);
– hepatotoxicity (see “Precautions”);
– risk of aneurysm and aortic dissection (see “Precautions”);
– serious adverse reactions with concomitant use of theophylline (see “Precautions”);
– diarrhea associated with Clostridium difficile (see “Precautions”);
– prolongation of the QT interval (see “Precautions”);
– disorders of the musculoskeletal system in children (see “Precautions”);
– photosensitivity/phototoxicity (see “Precautionary measures”);
– development of drug-resistant bacteria (see “Precautions”).
Clinical trial results
Because clinical trials are conducted under a different set of conditions, the incidence of adverse reactions observed in these clinical trials may not be the same as those obtained in other clinical trials and observed in clinical practice.
Adult patients
During clinical trials of oral and parenteral forms of ciprofloxacin, 49,038 patients were treated.
The most common adverse reactions reported in clinical trials (regardless of dosage, duration of therapy, indication) were nausea (2.5%), diarrhea (1.6%), abnormal liver function tests (1.3%), vomiting (1%), and rash (1%).
Clinically important adverse reactions that were observed in <1% of patients treated with ciprofloxacin are listed below.
Body as a whole: headache, pain, incl. abdominal pain/discomfort.
From the cardiovascular system: fainting, angina pectoris, myocardial infarction, cardiac and respiratory arrest, tachycardia, hypotension.
From the central nervous system: anxiety, dizziness, sleep disturbance, nightmares, hallucinations, paranoia, psychosis (toxic), manic reaction, irritability, tremor, ataxia, convulsions (including status epilepticus), malaise, anorexia, phobia, depersonalization, depression (can potentially lead to self-injurious behavior such as suicidal acts/ideations, and also attempted or successful suicide), paresthesia, abnormal gait, migraine.
From the gastrointestinal tract: intestinal perforation, gastrointestinal bleeding, cholestatic jaundice, hepatitis, pancreatitis.
From the hematopoietic and lymphatic systems: petechiae.
Metabolism and nutritional disorders: hyperglycemia, hypoglycemia.
From the musculoskeletal system: arthralgia, joint stiffness, muscle weakness.
From the genitourinary system: interstitial nephritis, renal failure.
From the respiratory system: dyspnea, laryngeal edema, hemoptysis, bronchospasm.
Skin/hypersensitivity: anaphylactic reactions, including potentially life-threatening anaphylactic shock, erythema multiforme/Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis, pruritis, urticaria, photosensitivity/phototoxicity reactions, flushing, fever, angioedema, erythema nodosum, sweating.
From the senses: blurred vision, visual impairment (chromatopsia and photopsia), decreased visual acuity, diplopia, tinnitus, hearing loss, loss of taste.
Pediatric patients
The short-term (6 weeks) and long-term (1 year) musculoskeletal and nervous system safety of ciprofloxacin, administered orally or intravenously, was compared with cephalosporin for the treatment of complicated urinary tract infections and pyelonephritis in pediatric patients aged 1 to 17 years (mean age (6±4) years) in an international multicenter study. Duration of therapy ranged from 10 to 21 days (median treatment duration 11 days, range 1 to 88 days). A total of 335 patients receiving ciprofloxacin and 349 patients receiving a comparator were included.
The Independent Pediatric Safety Committee (IPSC) reviewed all cases of musculoskeletal adverse reactions, including abnormal gait or joint abnormality on physical examination (at baseline or requiring treatment). Within 6 weeks of treatment, musculoskeletal adverse reactions were reported in 9.3% (31/335) of patients receiving ciprofloxacin, compared with 6% (21/349) of patients receiving the comparator drug. All musculoskeletal adverse reactions occurring within 6 weeks resolved (clinical resolution of signs and symptoms) usually within 30 days of completion of treatment. Radiological studies were not routinely used to confirm resolution of adverse reactions. Patients receiving ciprofloxacin were more likely to report more than one adverse reaction on more than one occasion compared with control patients. The incidence of musculoskeletal side effects was consistently higher in the ciprofloxacin group compared with the control group in all age subgroups. At the end of 1 year, the incidence of these adverse reactions reported at any time during this period was 13.7% (46/335) in the ciprofloxacin group compared with 9.5% (33/349) in the comparator group, as shown in Table 2.
Table 2
Adverse reactions from the musculoskeletal system1 as assessed by IPSC
Ciprofloxacin
Comparator drug
All patients (within 6 weeks)
31/335 (9.3%)
21/349 (6%)
95% CI2
(-0.8%, +7.2%)
Age group
12–24 months
1/36 (2.8%)
0/41
2–6 years
5/124 (4%)
3/118 (2.5%)
6–12 years
18/143 (12.6%)
12/153 (7.8%)
12–17 years old
7/32 (21.9%)
6/37 (16.2%)
All patients (within 1 year)
46/335 (13.7%)
33/349 (9.5%)
95% CI2
(-0.6%, + 9.1%)
1 Adverse effects included were arthralgia, abnormal gait, abnormal joint examination, sprains, leg pain, back pain, arthrosis, bone pain, pain, myalgia, arm pain, and decreased joint range of motion (knee, elbow, ankle, hip, wrist, shoulder).
2 The study was designed to demonstrate that the rate of arthropathy in the ciprofloxacin group did not exceed that in the control group by more than +6%. At both the 6-week and 1-year assessments, the 95% CI showed that it could not be concluded that the ciprofloxacin group had comparable results to the control group.
The incidence of neurological adverse reactions within 6 weeks of starting ciprofloxacin treatment was 3% (9/335) in the ciprofloxacin group compared with 2% (7/349) in the control group and included dizziness, nervousness, insomnia and somnolence.
In this study, the overall incidence of adverse reactions within 6 weeks of starting ciprofloxacin treatment was 41% (138/335) compared with 31% (109/349) in the comparison group. The most common adverse reactions were gastrointestinal – 15% (50/335) in patients receiving ciprofloxacin, compared with 9% (31/349) of patients in the comparison group.
Serious adverse reactions occurred in 7.5% (25/335) of patients receiving ciprofloxacin, compared with 5.7% (20/349) of control patients. Discontinuation of ciprofloxacin due to adverse effects occurred in 3% (10/335) of patients receiving ciprofloxacin, compared with 1.4% (5/349) of patients in the comparison group. Other adverse reactions that occurred in at least 1% of patients receiving ciprofloxacin were diarrhea (4.8%), vomiting (4.8%), abdominal pain (3.3%), dyspepsia (2.7%), nausea (2.7%), fever (2.1%), asthma (1.8%), and rash (1.8%).
Short-term safety data for ciprofloxacin were also collected in a randomized, double-blind clinical trial for acute pulmonary exacerbations in patients with cystic fibrosis (aged 5 to 17 years). Sixty-seven patients received IV ciprofloxacin 10 mg/kg every 8 hours for 1 week, followed by oral ciprofloxacin 20 mg/kg every 12 hours until 10 to 21 days of treatment were completed. Periodically, the condition of the musculoskeletal system was assessed by experts not related to the treatment. Patients were followed for an average of 23 days after completion of treatment (range 0–93 days). Musculoskeletal adverse reactions were reported in 22% of patients, decreased range of motion in 12% of patients, and arthralgia in 10% of patients. The effectiveness of ciprofloxacin for the treatment of acute pulmonary exacerbations in pediatric patients with cystic fibrosis has not been established.
In addition to the adverse reactions reported in pediatric patients in clinical trials, it is expected that adverse reactions reported in adults during clinical trials or post-marketing experience may also occur in pediatric patients.
Post-marketing experience
Listed below are adverse reactions that have been reported with fluoroquinolones, including ciprofloxacin. Because these cases are reported voluntarily, from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
From the cardiovascular system: prolongation of the QT interval, torsade de pointes, ventricular arrhythmia, vasculitis, hypertension.
From the central nervous system: myasthenia gravis, exacerbation of myasthenia gravis, peripheral neuropathy, polyneuropathy.
From the organ of vision: eye twitching, nystagmus.
From the gastrointestinal tract: pseudomembranous colitis.
From the hematopoietic and lymphatic system: pancytopenia (life-threatening or fatal), methemoglobinemia.
From the liver and biliary tract: liver failure (including fatal cases).
Infections and infestations: candidiasis (oral, gastrointestinal, vaginal).
Investigations: increased or decreased PT, increased cholesterol (serum), increased potassium levels (serum).
From the musculoskeletal system: myalgia, myoclonus, tendinitis, tendon rupture.
Mental disorders: agitation, confusion, delirium.
Skin disorders/hypersensitivity: acute generalized exanthematous pustulosis, fixed rashes; reactions similar to serum sickness, anosmia.
From the senses: hyperesthesia, hypoesthesia, loss of taste.
Laboratory parameters: liver – increased levels of ALT, AST, ALP, LDH, serum bilirubin; hematological – eosinophilia, leukopenia, decreased or increased platelet levels, pancytopenia; from the kidneys – increased levels of serum creatinine, urea nitrogen, crystalluria, cylindruria and hematuria have been reported; others are increased serum GGT levels, increased serum amylase levels, decreased blood glucose levels, increased uric acid levels, decreased Hb, anemia, bleeding diathesis, increased blood monocytes and leukocytosis.
Interaction
Interaction
Ciprofloxacin is an inhibitor of the cytochrome P450 isoenzyme CYP1A2. The simultaneous use of ciprofloxacin with other drugs metabolized primarily by CYP1A2 leads to an increase in the concentration of these drugs in the blood plasma and can lead to clinically significant adverse events.
Tizanidine. As a result of a pharmacokinetic study involving healthy volunteers, the simultaneous use of ciprofloxacin (500 mg 2 times a day for 3 days) and tizanidine (4 mg once) revealed a significant increase in the systemic exposure of tizanidine (Cmax 7 times, AUC 10 times). The simultaneous use of ciprofloxacin and tizanidine is contraindicated due to the potentiation of the hypotensive and sedative effects of tizanidine (see “Contraindications”).
Theophylline. The simultaneous use of ciprofloxacin and theophylline may lead to an increased risk of adverse reactions in the patient, incl. from the central nervous system. If simultaneous use cannot be avoided, it is recommended to continuously monitor serum theophylline concentrations and, if necessary, reduce the dose of theophylline (see “Precautions”).
Other xanthine derivatives. The simultaneous use of ciprofloxacin and caffeine or pentoxifylline can lead to decreased clearance, increased concentrations of xanthine derivatives in the blood serum and prolongation of serum T1/2. Caution should be used to monitor xanthine toxicity and adjust the dose if necessary.
Drugs that cause prolongation of the QT interval. Since ciprofloxacin may further prolong the QT interval, concomitant use of ciprofloxacin should be avoided in patients receiving drugs that cause QT interval prolongation (for example, class IA or class III antiarrhythmics, tricyclic antidepressants, macrolides, antipsychotics) (see “Precautions”).
Oral hypoglycemic agents. When using ciprofloxacin and oral hypoglycemic agents simultaneously, caution must be exercised due to the potentiation of the glucose-lowering effect. The development of severe hypoglycemia has been reported with the simultaneous use of ciprofloxacin and oral hypoglycemic agents, mainly sulfonylurea derivatives (for example, glibenclamide, glimepiride). There were no reports of deaths. Careful monitoring of blood glucose levels is necessary (see “Side effects”).
Phenytoin. With the simultaneous use of ciprofloxacin and phenytoin, a change (increase or decrease) in the content of phenytoin in the blood serum was observed. To avoid seizures associated with decreased phenytoin levels, and to prevent adverse events associated with phenytoin overdose when ciprofloxacin is discontinued, caution and monitoring of phenytoin therapy is recommended in patients taking ciprofloxacin, including determination of serum phenytoin levels throughout the period of concomitant use and for a short period after completion of combination therapy.
Cyclosporine. With simultaneous use of ciprofloxacin and cyclosporine, a transient increase in serum creatinine concentration was observed. Caution must be exercised and monitoring of renal function (in particular, determination of creatinine concentration in the blood).
Anticoagulants. The combined use of ciprofloxacin and anticoagulants may lead to an increase in their anticoagulant effect. The magnitude of this effect may vary depending on the infection, age and general condition of the patient, so it is difficult to assess the effect of ciprofloxacin on increasing the INR. Caution should be exercised and the PT and INR should be monitored frequently when ciprofloxacin is co-administered with oral anticoagulants (eg, warfarin), as well as for a short time after completion of combination therapy.
Methotrexate. With the simultaneous use of methotrexate and ciprofloxacin, the renal tubular transport of methotrexate may slow down, which may be accompanied by an increase in the concentration of methotrexate in the blood plasma. This may increase the likelihood of developing side effects of methotrexate. In this regard, caution should be exercised: patients receiving concomitant methotrexate and ciprofloxacin should be closely monitored.
Ropinirole. In a study of 12 patients with Parkinson’s disease who received 6 mg ropinirole once daily and 500 mg ciprofloxacin twice daily, the Cmax and AUC of ropinirole were increased by 60 and 84%, respectively. Caution should be exercised when using ropinirole and ciprofloxacin simultaneously. Monitoring for side effects of ropinirole and appropriate dose adjustment is recommended when coadministered with ciprofloxacin and for a short time after completion of combination therapy.
Clozapine. With simultaneous use of clozapine and ciprofloxacin at a dose of 250 mg for 7 days, an increase in serum concentrations of clozapine and N-desmethylclozapine was observed by 29 and 31%, respectively. Caution should be exercised, careful monitoring of clozapine-associated adverse reactions and, if necessary, adjustment of the clozapine dosage regimen during its combined use with ciprofloxacin and for a short time after completion of combination therapy.
NSAIDs. The combination of very high doses of quinolones and NSAIDs (excluding acetylsalicylic acid) can provoke seizures; must be used with caution.
Sildenafil. With simultaneous use in healthy volunteers of ciprofloxacin at a dose of 500 mg and sildenafil at a single oral dose of 50 mg, an approximately 2-fold increase in the Cmax and AUC of sildenafil was observed. In this regard, caution and monitoring of sildenafil toxicity is necessary.
Duloxetine. Clinical studies have shown that the simultaneous use of duloxetine and strong inhibitors of the CYP1A2 isoenzyme (such as fluvoxamine) can lead to an increase in the AUC and Cmax of duloxetine by 5 and 2.5 times, respectively. The simultaneous use of ciprofloxacin and duloxetine should be avoided. If this is not possible, monitoring for duloxetine toxicity is necessary.
Zolpidem. When used simultaneously with ciprofloxacin, the level of zolpidem in the blood may increase; it is recommended to avoid combined use.
Probenecid. Probenecid slows down the rate of ciprofloxacin excretion by the kidneys and increases its concentration in the blood serum. When using ciprofloxacin and probenecid simultaneously, caution should be exercised (the side effects of ciprofloxacin may increase).
Omeprazole. When ciprofloxacin was administered as a single dose of 1000 mg and omeprazole (40 mg once daily for 3 days) in 18 healthy volunteers, the AUC and Cmax of ciprofloxacin in the blood decreased by 20 and 23%, respectively. The clinical significance of this interaction has not been determined.
Lidocaine. A study involving 9 healthy volunteers showed that simultaneous intravenous administration of lidocaine at a dose of 1.5 mg/kg and ciprofloxacin (500 mg 2 times a day) led to an increase in Cmax and AUC of lidocaine by 12 and 26%, respectively. Despite the good tolerability of lidocaine, when used simultaneously with ciprofloxacin, side effects may increase due to interaction.
Histamine H2 receptor antagonists do not appear to have a significant effect on the bioavailability of ciprofloxacin.
Metronidazole. With simultaneous use, the concentrations of ciprofloxacin and metronidazole in the blood serum do not change.
Metoclopramide. Metoclopramide significantly accelerates the absorption of ciprofloxacin when taken orally, which reduces the time to reach Cmax in blood plasma. No significant effect on the bioavailability of ciprofloxacin was observed.
Cation-containing drugs. Simultaneous oral use of ciprofloxacin and cation-containing drugs, such as multivitamins, magnesium- and aluminum-containing antacids, sucralfate, polymeric phosphate binders (for example, sevelamer, lantanan carbonate) and drugs with a large buffer capacity (for example, didanosine), mineral supplements containing calcium, iron, zinc – reduces absorption ciprofloxacin, which leads to a decrease in its level in blood serum and urine.
Concomitant use of antacids containing magnesium hydroxide or aluminum hydroxide can reduce the bioavailability of ciprofloxacin by 90%.
In the above cases, ciprofloxation should be taken either 2 hours before or 6 hours after taking such drugs.
Eating food and dairy products. The simultaneous use of oral ciprofloxacin and dairy products or drinks fortified with minerals (eg milk, yogurt, calcium-fortified juices) should be avoided as the absorption of ciprofloxacin may be reduced. Calcium contained in regular food does not significantly affect the absorption of ciprofloxacin.
Overdose
Overdose
Symptoms: In cases of acute overdose, reversible renal toxicity has been reported in some cases.
Treatment: induction of vomiting, gastric lavage, taking antacids containing magnesium, aluminum, calcium to reduce the absorption of ciprofloxacin. Careful monitoring of the patient’s condition and symptomatic therapy, including monitoring of renal function, urine pH, and, if necessary, acidification, should be carried out to prevent the development of crystalluria. It is necessary to ensure sufficient fluid intake. Only a small (<10%) amount of ciprofloxacin can be removed by hemo- or peritoneal dialysis.
Storage conditions
Storage conditions
In a place protected from moisture, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Sun Pharmaceutical Industries Ltd, India
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a moisture-proof place, at a temperature not exceeding 25 °C |
| Manufacturer | Sun Pharmaceutical Industries Ltd, India |
| Medication form | sustained release tablets |
| Brand | Sun Pharmaceutical Industries Ltd |
Other forms…
Related products
Buy Cifran OD, 1000 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.