No products in the cart.
Cifran, 500 mg 10 pcs
€3.28 €2.98
Description
Pharmgroup:
The antimicrobial agent is a fluoroquinolone.
Pharmic action:
Cyfran, like other fluorinated quinolones, blocks bacterial DNA gyrase, thus suppressing the DNA function of the bacteria. Cifran is active against Gram-positive and Gram-negative pathogens, including strains resistant to penicillins, cephalosporins and and/or aminoglycosides. The spectrum of action of Cifran covers such microorganisms:
– Aerobic Gram-negative bacteria – Escherichia coli, Klebsiella spp., Salmonella spp., Proteus spp., Shigella spp., Yersinia spp., Enterobacter spp, Morganella morganii, Providencia spp., Vibrio spp., Citrobacter spp., Serratia spp., Campylobacter spp., Pseudomonas aeruginosa, P. cepacia, Neisseria gonorrhoeae, N. meningitidis, Haemophilus influenzae, H. ducreyi, Acinetobacter spp, Moraxella catarrhalis, Gardnerella vaginalis, Pasteurella multocida, Helicobacter pylori;
– aerobic gram-positive bacteria – staphylococci, including strains that produce penicillinase and strains resistant to methicillin, streptococci, including Streptococcus pneumoniae, Listeria monocytogenes, Corynebacterium spp.
The anaerobic bacteria, mycobacteria, mycoplasmas, rickettsia, chlamydia, Nocardia asteroides, Ureaplasma urealyticum, pale spirochete, viruses, fungi and protozoa are not sensitive to the drug.
Indications
Indications
Urinary tract infections, gonorrhea, pneumonia, skin and soft tissue infections, bone and joint infections, intestinal infections, barley, blood poisoning.
Pharmacological effect
Pharmacological effect
Pharmaceutical group:
antimicrobial agent – fluoroquinolone.
Pharmaceutical action:
Cifran, like other fluorinated quinolones, blocks bacterial DNA gyrase, as a result of which it suppresses the function of bacterial DNA. Cifran is active against gram-positive and gram-negative pathogens, including strains resistant to penicillins, cephalosporins and/or aminoglycosides. The spectrum of action of Tsifran covers the following microorganisms:
— aerobic gram-negative bacteria – Escherichia coli, Klebsiella spp., Salmonella spp., Proteus spp., Shigella spp., Yersinia spp., Enterobacter spp., Morganella morganii, Providencia spp., Vibrio spp., Citrobacter spp., Serratia spp., Campylobacter spp., Pseudomonas aeruginosa, P. cepacia, Neisseria gonorrhoeae, N. meningitidis, Haemophilus influenzae, H. ducreyi, Acinetobacter spp., Moraxella catarrhalis, Gardnerella vaginalis, Pasteurella multocida, Helicobacter pylori;
— aerobic gram-positive bacteria – staphylococci, including strains that produce penicillinase and strains resistant to methicillin, streptococci, including Streptococcus pneumoniae, Listeria monocytogenes, Corynebacterium spp.
The following are insensitive to the drug: anaerobic bacteria, mycobacteria, mycoplasma, rickettsia, chlamydia, Nocardia asteroides, Ureaplasma urealyticum, spirochete pallidum, viruses, fungi and protozoa.
Special instructions
Special instructions
Ciprofloxacin, like other drugs in this class, has been found to cause arthropathy of large joints in animals. When analyzing the current data on the safety of the use of ciprofloxacin in children under 18 years of age, most of whom have cystic fibrosis of the lungs, no connection was established between damage to cartilage or joints with taking the drug. It is not recommended to use ciprofloxacin in children for the treatment of diseases other than the treatment of complications of cystic fibrosis of the lungs (in children from 5 to 17 years old) associated with Pseudomonas aeruginosa and for the treatment and prevention of the pulmonary form anthrax (after suspected or proven infection with Bacillus anthracis).
In the outpatient treatment of patients with pneumonia caused by bacteria of the genus Pneumococcus, ciprofloxacin should not be used as the first choice drug.
In some cases, adverse reactions from the central nervous system may occur after the first use of the drug. In very rare cases, psychosis can manifest itself as suicide attempts. In these cases, the use of ciprofloxacin should be discontinued immediately.
For patients with epilepsy, a history of seizures, vascular diseases and organic brain damage, due to the risk of developing side effects from the central nervous system, ciprofloxacin should be prescribed only for “vital indications”, in cases where the expected clinical effect outweighs the possible risk of side effects of the drug.
If severe or prolonged diarrhea occurs during or after treatment with ciprofloxacin, the diagnosis of pseudomembranous colitis should be excluded, which requires immediate discontinuation of the drug and the appointment of appropriate treatment.
The use of drugs that suppress intestinal motility is contraindicated. Patients, especially those with liver disease, may experience cholestatic jaundice, as well as a temporary increase in the activity of “liver” transaminases and alkaline phosphatase.
Compliance with the appropriate dosage regimen is required when prescribing the drug to patients with renal and hepatic insufficiency.
Sometimes, after taking the first dose of ciprofloxacin, allergic reactions may occur, and rarely, anaphylactic shock. In these cases, ciprofloxacin should be stopped immediately and appropriate treatment administered.
In elderly patients previously treated with corticosteroids, cases of Achilles tendon rupture may occur.
If pain occurs in the tendons or when the first signs of tendinitis appear, treatment should be stopped due to the fact that isolated cases of inflammation and even tendon rupture have been described during treatment with fluoroquinolones.
During treatment with ciprofloxacin, it is necessary to avoid contact with direct sunlight, since photosensitivity reactions may occur when taking ciprofloxacin. Treatment should be discontinued if symptoms of photosensitivity are observed (for example, skin changes resembling sunburn).
Ciprofloxacin is known to be a moderate inhibitor of the CYP1A2 isoenzyme.
Caution should be exercised when simultaneous use of ciprofloxacin and drugs metabolized by this isoenzyme, such as theophylline, methylxanthine, caffeine, because Increased serum concentrations of these drugs may cause associated side effects.
To avoid the development of crystalluria, it is unacceptable to exceed the recommended daily dose; it is also necessary to have sufficient fluid intake (while maintaining normal diuresis) and maintaining an acidic urine reaction.
For genital infections suspected of being caused by fluoroquinolone-resistant Neisseria gonorrhoeae strains, local information on ciprofloxacin resistance should be taken into account and susceptibility testing should be confirmed in laboratory tests.
Impact on the ability to drive vehicles and machinery:
Patients taking ciprofloxacin should be careful when driving a car and engaging in other potentially hazardous activities that require increased attention and speed of psychomotor reactions.
Active ingredient
Active ingredient
Ciprofloxacin
Composition
Composition
Active ingredients:
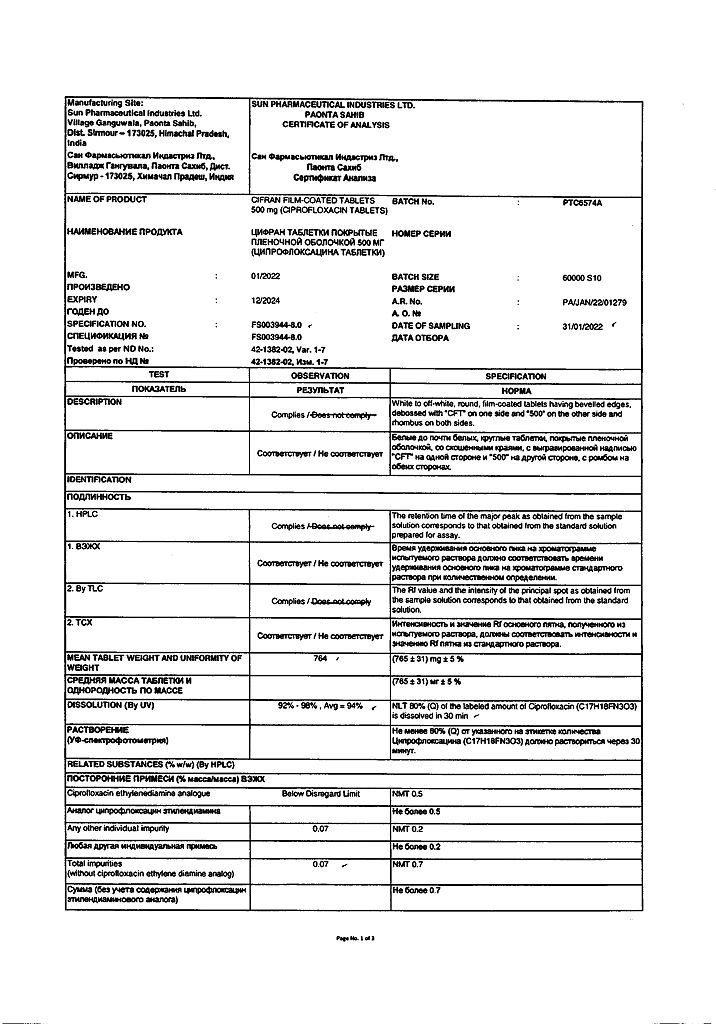
ciprofloxacin hydrochloride 594.14 mg, equivalent to ciprofloxacin 500 mg.
Excipients:
microcrystalline cellulose 50.08 mg,
corn starch 36.62 mg,
magnesium stearate 7.48 mg,
purified talc 4.56 mg,
colloidal anhydrous silicon 9.36 mg,
sodium starch glycolate 47.76 mg,
purified water* q.s.
Film shell material:
Opadray-ОY-858910 white 26.88 mg, purified talc 2.44 mg, purified talc q.s., purified water.
Contraindications
Contraindications
Hypersensitivity to the drug Cifran, severe renal dysfunction, epilepsy.
Side Effects
Side Effects
From the digestive system:
Common: nausea, diarrhea.
Uncommon: abdominal pain; vomit; flatulence; anorexia; changes in liver test parameters – alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase; jaundice; hyperbilirubinemia.
Rarely: oral candidiasis, jaundice, including cholestatic, pseudomembranous colitis.
Very rare: candidiasis, hepatitis, liver tissue necrosis (in extremely rare cases progressing to life-threatening liver failure), life-threatening pseudomembranous colitis with possible fatal outcome, pancreatitis.
From the skin:
Uncommon: rash.
From the hematopoietic system:
Uncommon: eosinophilia.
Rarely: anemia, leukopenia (granulocytopenia), leukocytosis, increased or decreased prothrombin, thrombocytopenia, thrombocytosis, neutropenia. Very rare: hemolytic anemia, pancytopenia (including life-threatening), agranulocytosis, in extremely rare cases, life-threatening bone marrow suppression.
From the urinary system:
Uncommon: increased creatinine and urea nitrogen levels.
From the musculoskeletal system:
Uncommon: arthralgia.
Rarely: myalgia (muscle pain), joint swelling.
Very rare: myasthenia gravis, tendonitis (mainly Achilles tendons), partial or complete rupture of tendons (mainly Achilles), exacerbation of myasthenia gravis symptoms, arthritis, muscle weakness, exacerbation of myasthenia gravis symptoms.
From the central nervous system:
Uncommon: headache, dizziness, sleep disturbances, anxiety, confusion.
Rarely: migraine, hallucinations, sweating, paresthesia (including peripheral paralgesia), anxiety, nightmares, depression, tremor, convulsions, hyperesthesia, agitation, disorientation, dysesthesia, hypoesthesia, vertigo.
Very rare: grand mal seizures, unsteady gait, psychosis, increased intracranial pressure, ataxia, increased muscle tone, muscle cramps, impaired coordination of movements.
Frequency unknown: peripheral neuropathy and polyneuropathy.
From the skin:
Uncommon: pruritus, urticaria, maculopapular rash.
Very rare: petechiae, erythema multiforme, erythema nodosum, persistent skin rash.
From the senses:
Uncommon: taste disturbance.
Rarely: tinnitus, temporary hearing impairment, visual impairment (diplopia, color vision impairment), hearing loss.
Very rare: parosmia, anosmia.
Others:
Uncommon: asthenia (feeling of weakness, fatigue), candidiasis.
Rarely: peripheral edema, hyperglycemia, pain in the extremities, back pain, chest pain.
Very rare: increased activity of amylase, lipase.
From the cardiovascular system:
Rarely: tachycardia, feeling of a “rush” of blood to the face, decreased blood pressure, fainting, vasodilation.
Very rare: vasculitis.
Frequency unknown: QT interval prolongation, ventricular arrhythmias (including torsade de pointes).
Allergic reactions:
Rarely: anaphylactic reactions, fever, angioedema.
Very rare: anaphylactic shock, skin rash, serum sickness-like reactions, Stevens-Johnson syndrome (malignant exudative erythema), Lyell’s syndrome (toxic epidermal necrolysis).
From the respiratory system:
Rarely: dyspnea, laryngeal edema, respiratory distress (including bronchospasm).
From the skin:
Rarely: photosensitivity reactions.
From the urogenital system:
Rarely: acute renal failure, renal dysfunction, vaginal candidiasis, hematuria, crystalluria, interstitial nephritis.
In addition, the following adverse events were noted: cerebral artery thrombosis, polyuria, albuminuria, urinary retention. The connection between these adverse events and the use of ciprofloxacin has not been reliably confirmed.
Interaction
Interaction
When didanosine is used simultaneously with ciprofloxacin, the effect of ciprofloxacin is reduced due to the formation of complexes of ciprofloxacin with the aluminum and magnesium salts contained in didanosine.
Concomitant use of ciprofloxacin with theophylline may result in increased plasma theophylline concentrations due to competitive inhibition at cytochrome P450 binding sites, resulting in an increase in the elimination half-life of theophylline and an increased risk of theophylline-associated toxicity.
Concomitant use of sucralfate, antacids, drugs with a large buffer capacity (for example, antiretroviral drugs), as well as drugs containing aluminum, zinc, iron or magnesium ions, may cause a decrease in the absorption of ciprofloxacin, so ciprofloxacin should be taken either 1-2 hours before or 4 hours after taking these drugs.
This limitation does not apply to antacids belonging to the H2-receptor blocker class.
Concomitant use of ciprofloxacin with dairy products or mineral-fortified beverages (eg, milk, yogurt, calcium-fortified orange juice) should be avoided as the absorption of ciprofloxacin may be reduced. However, calcium contained in other foods does not significantly affect the absorption of ciprofloxacin.
With the combined use of ciprofloxacin and omeprazole, a slight decrease in the maximum concentration (Cmax) of the drug in the blood plasma and a decrease in the area under the concentration-time curve (AUC) may be observed.
The combination of very high doses of quinolones (gyrase inhibitors) and some non-steroidal anti-inflammatory drugs (except acetylsalicylic acid) can provoke seizures.
With the simultaneous use of ciprofloxacin and anticoagulants (including warfarin), bleeding time is prolonged.
With the simultaneous use of ciprofloxacin and cyclosporine, the nephrotoxic effect of the latter increases. With simultaneous therapy with ciprofloxacin and cyclosporine, a short-term increase in plasma creatinine concentration was observed. In such cases, it is necessary to determine the concentration of creatinine in the blood twice a week.
In some cases, the simultaneous use of ciprofloxacin and glibenclamide may enhance the effect of glibenclamide (hypoglycemia).
Co-administration of uricosuric drugs, including probenecid, slows down the rate of excretion of ciprofloxacin by the kidneys (up to 59%) and increases the concentration of ciprofloxacin in the blood plasma.
With simultaneous administration of ciprofloxacin, the tubular transport (renal metabolism) of methotrexate may slow down, which may be accompanied by an increase in the concentration of methotrexate in the blood plasma. This may increase the likelihood of side effects from methotrexate. In this regard, patients receiving concomitant therapy with methotrexate and ciprofloxacin should be closely monitored.
Metoclopramide accelerates the absorption of ciprofloxacin, reducing the period of time required to achieve its maximum plasma concentration. However, the bioavailability of ciprofloxacin does not change.
As a result of a clinical study involving healthy volunteers, the simultaneous use of ciprofloxacin and tizanidine revealed an increase in the concentration of tizanidine in the blood plasma: an increase in Cmax by 7 times (from 4 to 21 times), an increase in AUC by 10 times (from 6 to 24 times). Hypotensive and sedative side effects are associated with increased serum concentrations of tizanidine. Therefore, the simultaneous use of ciprofloxacin and tizanidine is contraindicated.
Ciprofloxacin can be used in combination with other antibiotics. As shown in in vitro studies, the combined use of ciprofloxacin and β-lactam antibiotics, as well as aminoglycosides, was accompanied by a predominantly additive and indifferent effect; Relatively rarely, an increase in the effect of both drugs was observed, and very rarely, a weakening was observed.
Overdose
Overdose
In cases of oral overdose, reversible toxic effects on the renal parenchyma have been observed in several cases. Therefore, in case of overdose, in addition to standard measures (gastric lavage, use of emetics, administration of large amounts of fluid, creation of an acidic urine reaction), it is also recommended to monitor kidney function and take magnesium- and calcium-containing antacids, which reduce the absorption of ciprofloxacin.
A specific antidote is not known. It is necessary to carefully monitor the patient’s condition, perform gastric lavage, carry out the usual emergency measures, and ensure sufficient fluid intake. Using hemo- and peritoneal dialysis, only a small amount (less than 10%) of the drug can be removed.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C, in sealed packaging
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Sun Pharmaceutical Industries Ltd, India
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C, in a sealed package |
| Manufacturer | Sun Pharmaceutical Industries Ltd, India |
| Medication form | pills |
| Brand | Sun Pharmaceutical Industries Ltd |
Related products
Buy Cifran, 500 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.