No products in the cart.
Description
Pharmacodynamics
Ifosfamide is an alkylating cytostatic from the group of nitrogenous mustard, a derivative of oxazaphosphorins.
The antitumor activity of ifosfamide is conditioned by alkylation of nucleophilic centers, disruption of DNA synthesis and blockage of mitotic division of tumor cells.
DNA damage occurs most frequently in G1 and G2 phases of the cell cycle.
Pharmacokinetics
After IV administration, the active substance, which is a prodrug (inactive transport form), is metabolized to the pharmacologically active metabolite 4-hydroxyphosphamide.
Activated by enzymes (phosphoamidases) of the liver and tumor tissue.
In patients with impaired liver function, activation is delayed or even reduced.
After a single IV administration of 5 g/m2 the plasma concentration decreases bioexponentially, with a T1/2 end phase of 15 h and excretion of 61% of the dose in unchanged form; at lower doses (1.6-2.4 g/m2) excretion is monoexponential, with T1/2 about 7 h, and the proportion of unchanged drug in urine is reduced 4-5 times (12-18% of dose).
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
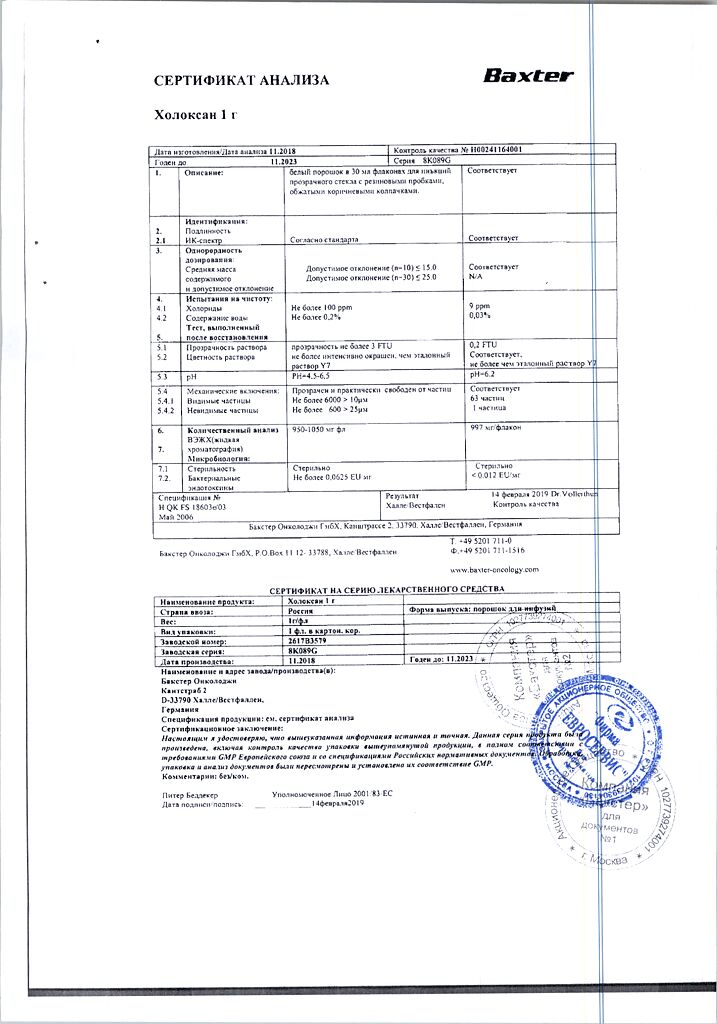
1 vial contains:
Active substances:
ifosfamide 1 g.
In a vial 1 g of powder.
There is 1 vial in the carton.
How to take, the dosage
How to take, the dosage
Ifosfamide is part of many chemotherapeutic regimens; therefore, the data in the literature should be used to guide the choice of regimen and doses in each individual case.
The drug is administered by IV drip for 30 minutes or as a 24-hour infusion. A solution with a concentration not exceeding 4% is used.
To reduce the likelihood of hemorrhagic cystitis, mesna is used concomitantly with ifosfamide at a total dose of 60% of the ifosfamide dose.
Preparation of solution for intravenous administration
Powder in vials is dissolved in water for injection to obtain a concentration of 40 mg/1 ml.
For intravenous administration within 30 minutes the obtained solution is diluted in 500 ml of 0.9% sodium chloride solution, Ringer’s solution or 5% dextrose solution.
In order to administer the drug as a 24-hour infusion, the resulting drug solution is diluted in 3 liters of 0.9% sodium chloride solution or 5% dextrose solution. Ifosfamide and mesna can be mixed in the same infusion solution.
Interaction
Interaction
When concomitant use with drugs causing myelotoxic, neurotoxic and nephrotoxic effects, an increased side effect is possible.
When concomitant use with inducers of microsomal liver enzymes an increase in the formation of alkylating metabolites is possible.
When used concomitantly, it increases the hypoglycemic effect of antidiabetic drugs.
Allopurinol increases myelosuppression.
Mesna reduces nephrotoxicity.
Ifosfamide may increase the skin response to radiation.
The concomitant administration of warfarin may decrease blood clotting and increase the risk of bleeding.
Special Instructions
Special Instructions
Before the start of treatment it is necessary to sanitize foci of chronic infection and correct possible electrolyte imbalances.
When treating with the drug it is necessary to monitor the peripheral blood picture regularly (especially paying attention to the number of neutrophils and thrombocytes), laboratory parameters of liver and kidney function, as well as to analyze urine regularly for the presence of red blood cells, the appearance of which can precede the development of hemorrhagic cystitis.
Women and men should use reliable contraception during treatment and for 3 months after therapy with ifosfamide.
In patients with impaired renal function and urine flow, the incidence of toxic effects of the drug on the CNS may increase, which may require reducing the dose of ifosfamide.
In order to ensure excretion of uric acid, patients should consume sufficient fluids.
If the first signs of bladder inflammation or blood in the urine occur, therapy with ifosfamide should be stopped.
The treatment with ifosfamide may suppress natural defense mechanisms, and antibody production in the patient’s body may decrease in response to administration of vaccines.
Impact on ability to drive vehicles and other mechanisms requiring high concentration
When treating with Holoxan there may be nausea and vomiting and encephalopathy which may affect the ability to drive or operate other mechanisms. Therefore, you should abstain from driving a vehicle or other means of transport. You should also avoid working with electrical tools and machinery.
Contraindications
Contraindications
With caution: hypoproteinemia, hypoalbuminemia, electrolyte imbalance, advanced age, immunosuppression, diabetes, chronic hepatic insufficiency, brain metastases, cerebral symptoms, varicella (including recent or recent cases of chickenpox).
Infectious diseases such as varicella (including recent or after exposure to the disease), herpes zoster, and acute infectious diseases.
Side effects
Side effects
Blood system: leukopenia, thrombocytopenia, anemia. The lowest number of leukocytes and platelets is observed in 7-14 days, the recovery of the blood picture occurs usually in 21 days after the end of the course.
In the digestive system: nausea and vomiting; rarely – stomatitis, liver dysfunction, usually manifested by increased liver enzymes activity and/or serum bilirubin level.
Urinary system disorders: hemorrhagic cystitis, dysuria, frequent urination and other symptoms of bladder inflammation (blood in the urine, painful urination), renal disorders (increased concentration of creatinine and urea in serum, decreased creatinine clearance. glucosuria). Proteinuria and metabolic acidosis may also be observed.
CNS disorders: disorientation, confusion, hallucinations, fatigue, agitation, encephalopathy; less frequently – dizziness; rarely – seizures, coma, peripheral polyneuropathy.
Reproductive system disorders: disorders of gland function (azoospermia, amenorrhea).
Skin and skin appendages: reversible alopecia, photosensitization.
Local reactions: redness, swelling or pain at the injection site.
Others: cardiotoxic effects, immunosuppression, infectious complications, slower wound healing rate, pulmonary symptoms (cough or shortness of breath), increased body temperature, allergic reactions.
Overdose
Overdose
Symptoms: more rapid development and acute severity of the main side effects.
Treatment: symptomatic, with mandatory use of Mesna.
Pregnancy use
Pregnancy use
The drug is contraindicated in pregnancy and lactation.
Additional information
| Weight | 0.052 kg |
|---|---|
| Shelf life | 5 years. The prepared solution should be used within 24 hours. |
| Conditions of storage | Store at temperatures not exceeding 25 ° C in places out of the reach of children. |
| Manufacturer | Baxter Oncology GmbH, Germany |
| Medication form | Powder for preparation of solution for infusion |
| Brand | Baxter Oncology GmbH |
Related products
Buy Choloxane, powder 1 g with delivery to USA, UK, Europe and over 120 other countries.