No products in the cart.
Description
Pharmacodynamics
Ifosfamide is an alkylating cytostatic from the group of nitrogenous mustard, a derivative of oxazaphosphorins. The antitumor activity of ifosfamide is caused by alkylation of nucleophilic centers, disruption of DNA synthesis and blockage of mitotic division of tumor cells. DNA damage occurs most frequently in G1 and G2 phases of the cell cycle.
Pharmacokinetics
After IV administration, the active substance, which is a prodrug (inactive transport form), is metabolized to the pharmacologically active metabolite 4-hydroxyyphosphamide. It is activated by enzymes (phosphoamidases) of the liver and tumor tissue. In patients with liver dysfunction the activation is delayed or even reduced.
After a single IV administration of 5 g/m2, the plasma concentration decreases bioexponentially, with a T1/2 end phase of 15 h and excretion of 61% of the dose in unchanged form; At lower doses (1.6-2.4 g/m2) excretion is monoexponential, with a T1/2 of about 7 hours, and the proportion of unchanged drug in urine is reduced 4-5 times (12-18% of dose).
< br>.
Indications
Indications
Germ cell tumors;
ovarian cancer;
malignant testicular tumors;
lung cancer;
breast cancer;
pancreatic cancer;
endometrial cancer;
cervical cancer;
malignant lymphomas;
soft tissue sarcomas;
osteogenic sarcomas;
Wilms tumor;
Ewing’s sarcoma.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Ifosfamide is an alkylating cytostatic from the nitrogen mustard group, a derivative of oxazaphosphorines. The antitumor activity of ifosfamide is due to alkylation of nucleophilic centers, disruption of DNA synthesis and blocking the mitotic division of tumor cells. DNA damage occurs most frequently during the G1 and G2 phases of the cell cycle.
Pharmacokinetics
After intravenous administration, the active substance, which is a prodrug (inactive transport form), is metabolized into the pharmacologically active metabolite 4-hydroxyphosphamide. Activated by enzymes (phosphoamidase) of the liver and tumor tissue. In patients with impaired liver function, activation is slowed down and even reduced.
After a single intravenous injection of 5 g/m2, the plasma concentration decreases bioexponentially, with T1/2 of the final phase being 15 hours and 61% of the dose being excreted unchanged; at lower doses (1.6-2.4 g/m2), excretion proceeds monoexponentially, with T1/2 of about 7 hours, while the proportion of unchanged drug in the urine decreases by 4-5 times (12-18% of the dose).
Special instructions
Special instructions
Before starting treatment, it is necessary to sanitize foci of chronic infection and correct possible electrolyte imbalances.
During treatment with the drug, it is necessary to regularly monitor the peripheral blood picture (especially paying attention to the number of neutrophils and platelets), laboratory indicators of liver and kidney function, and also regularly conduct a urine test for the presence of red blood cells, the appearance of which may precede the development of hemorrhagic cystitis.
Women and men should use reliable methods of contraception during treatment and for 3 months after the end of ifosfamide therapy.
If renal function and urine outflow are impaired, the frequency of toxic effects of the drug on the central nervous system may increase, and therefore it may be necessary to reduce the dose of ifosfamide.
To ensure the elimination of uric acid, patients should consume sufficient fluids.
Ifosfamide therapy should be discontinued at the first sign of bladder inflammation or blood in the urine.
When treated with ifosfamide, natural defense mechanisms may be suppressed, and the production of antibodies in the patient’s body in response to vaccines may decrease.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
During therapy with Holoxan, nausea and vomiting, as well as encephalopathy, may occur, which may affect the ability to drive a car or operate other mechanisms. Therefore, you should refrain from driving a car or other vehicles. You should also not work with electrical tools and machinery.
Active ingredient
Active ingredient
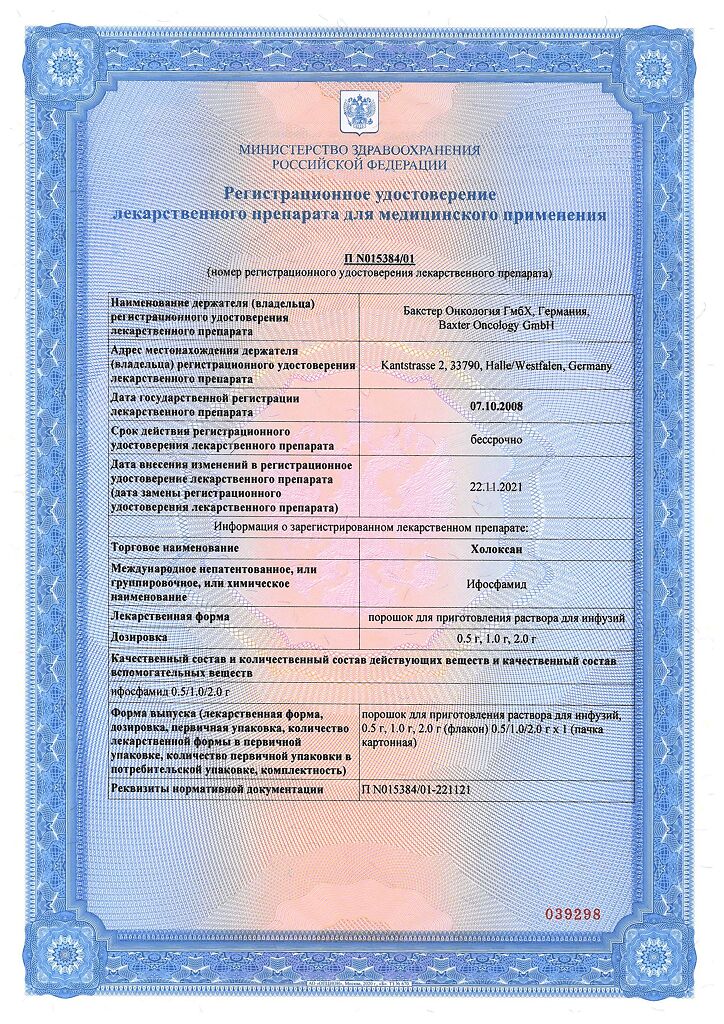
Ifosfamide
Composition
Composition
1 bottle contains:
Active ingredients:
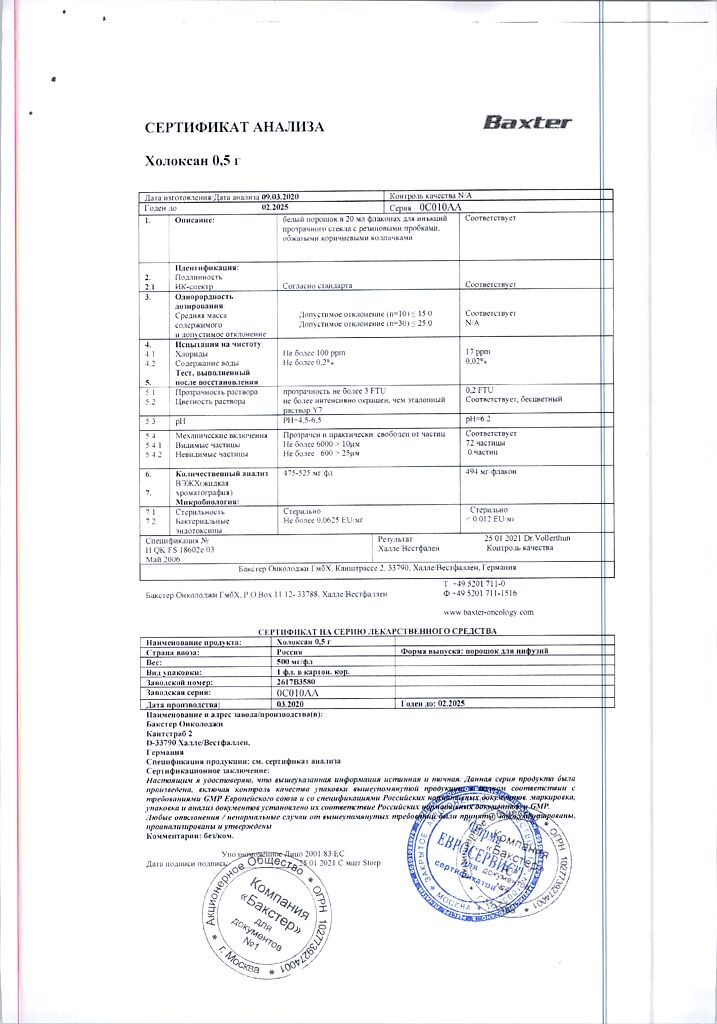
ifosfamide 500 mg.
The bottle contains 0.5 g of powder.
There is 1 bottle in a cardboard package.
Pregnancy
Pregnancy
The drug is contraindicated during pregnancy and lactation.
Contraindications
Contraindications
Severe suppression of bone marrow function;
severe renal dysfunction;
obstruction of the urinary tract;
cystitis;
pregnancy;
lactation;
hypersensitivity to ifosfamide.
With caution: hypoproteinemia, hypoalbuminemia, electrolyte imbalance, old age, immunosuppression, diabetes mellitus, chronic liver failure, brain metastases, cerebral symptoms, chickenpox (including recent or after contact with sick people), herpes zoster, acute infectious diseases.
Side Effects
Side Effects
From the hematopoietic system: leukopenia, thrombocytopenia, anemia. The lowest level of leukocytes and platelets is observed after 7-14 days; the blood picture is restored usually 21 days after the end of the course.
From the digestive system: nausea and vomiting; rarely – stomatitis, liver dysfunction, usually manifested as increased activity of liver enzymes and/or bilirubin levels in the blood serum.
From the urinary system: hemorrhagic cystitis, dysuria, frequent urination and other symptoms of inflammation of the bladder (blood in the urine, painful urination), impaired renal function (increased concentrations of creatinine and urea in the blood serum, decreased creatinine clearance, glucosuria). Proteinuria and metabolic acidosis may also occur.
From the central nervous system: disorientation, confusion, hallucinations, increased fatigue, agitation, encephalopathy; less often – dizziness; rarely – seizures, coma, peripheral polyneuropathy.
From the reproductive system: dysfunction of the gonads (azoospermia, amenorrhea).
From the skin and skin appendages: reversible alopecia, photosensitivity.
Local reactions: redness, swelling or pain at the injection site.
Other: cardiotoxic effects, immunosuppression, infectious complications, slowing the rate of wound healing, pulmonary symptoms (cough or shortness of breath), increased body temperature, allergic reactions.
Interaction
Interaction
When used simultaneously with drugs that cause myelotoxic, neurotoxic and nephrotoxic effects, increased side effects may occur.
When used together with inducers of microsomal liver enzymes, an increase in the formation of alkylating metabolites is possible.
When used simultaneously, it enhances the hypoglycemic effect of antidiabetic drugs.
Allopurinol enhances myelosuppression.
Mesna reduces nephrotoxicity.
Ifosfamide may increase the skin’s reaction to radiation.
Concomitant use of warfarin may reduce blood clotting and increase the risk of bleeding.
Overdose
Overdose
Symptoms: faster development and sharper severity of the main side effects.
Treatment: symptomatic, with mandatory use of Mesna.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25°C out of the reach of children.
Store the prepared solution at a temperature not exceeding 8°C.
Shelf life
Shelf life
5 years.
The prepared solution must be used within 24 hours.
Manufacturer
Manufacturer
Baxter Oncology GmbH, Germany
Additional information
| Shelf life | 5 years. The prepared solution should be used within 24 hours. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C in places out of the reach of children. Store the prepared solution at the temperature not more than 8°C. |
| Manufacturer | Baxter Oncology GmbH, Germany |
| Medication form | Powder for preparation of solution for infusion |
| Brand | Baxter Oncology GmbH |
Related products
Buy Choloxane, powder 0.5 g with delivery to USA, UK, Europe and over 120 other countries.