No products in the cart.
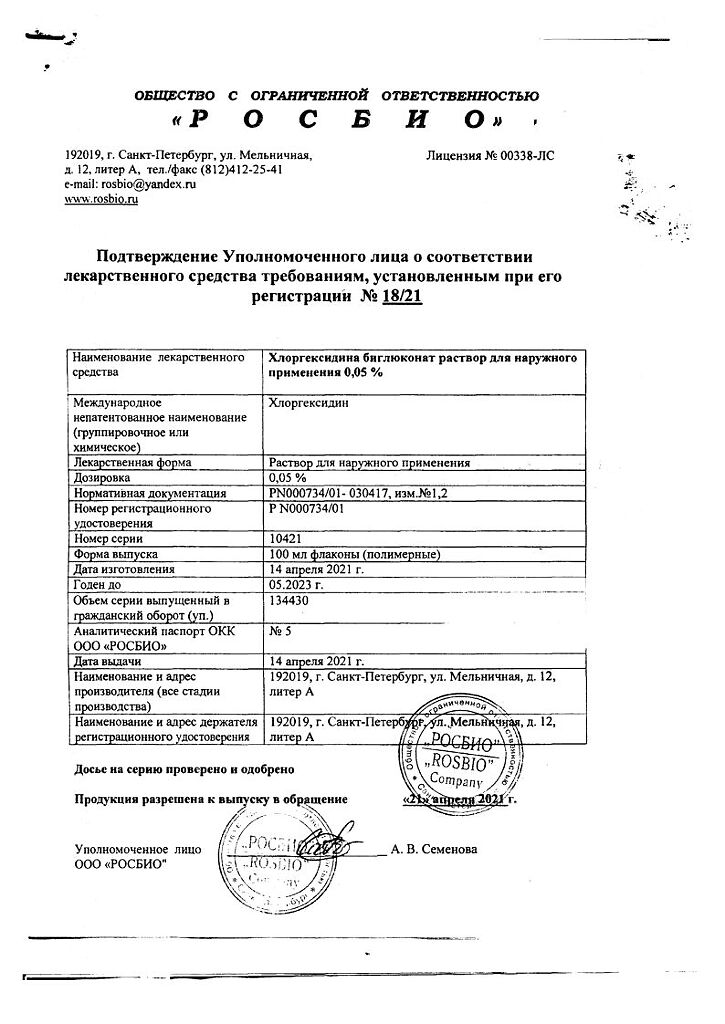
Chlorhexidine, 0.05% solution 100 ml
€0.52 €0.47
Description
Chlorhexidine has antimicrobial activity against Gram-negative and Gram-positive bacteria (Treponema spp., Neisseia gonorrhoeae, Tricyomonas spp, Chlamidia spp.), pathogens of nosocomial infections and tuberculosis, infections of viral etiology (hepatitis viruses, HIV, herpes, rotavirus gastroenteritis, enterovirus infections, influenza and other respiratory and viral infections), yeast-like fungi of the genus Candida, dermatophytes.
Prolonged action – up to 4 hours.
Pharmacokinetics
Systemic absorption with intravaginal use is negligible.
Indications
Indications
As a therapeutic and prophylactic agent for various infections, for antiseptic treatment and disinfection. Prevention of sexually transmitted infections (chlamydia, ureaplasmosis, trichomoniasis, gonorrhea, syphilis, genital herpes – use no later than 2 hours after sexual intercourse); disinfection of skin (abrasions, cracks). Purulent wounds, infected burns, bacterial and fungal diseases of the skin and mucous membranes in surgery, urology, obstetrics and gynecology, dentistry (gingivitis, stomatitis, aphthae, periodontitis, alveolitis).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antiseptic.
ATX code D08AC02
Pharmacological properties
Pharmacodynamics
Antiseptic agent, exhibits a bactericidal effect (against gram-positive and gram-negative bacteria) – at a temperature of 220C and
exposure for 1 min; fungicidal effect at a temperature of 220C and exposure for 10 minutes; virucidal effect (against lipophilic viruses). Effective against gram-positive and gram-negative bacteria (Treponema spp., Neisseria gonorrhoeae., Ureaplasma spp., Bacteroides fragilis., Chlamydia spp.); protozoa (Thrichomonas vaginalis); viruses and fungi. Bacterial spores are only affected at elevated temperatures. It is stable, after treatment of the skin it remains on it in a certain amount sufficient to exhibit a bactericidal effect. Retains activity (albeit somewhat reduced) in the presence of blood, pus, various secretions and organic substances.
Pharmacokinetics
The drug is not adsorbed into the systemic bloodstream when applied externally and does not have a systemic effect. Practically not absorbed from the gastrointestinal tract.
Special instructions
Special instructions
In case of contact with the mucous membranes of the eye, they should be rinsed quickly and thoroughly with water.
Impact on the ability to operate vehicles using machinery
The use of the drug does not affect the ability to perform potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions (driving vehicles, working with moving mechanisms, working as a dispatcher and operator).
Active ingredient
Active ingredient
Chlorhexidine
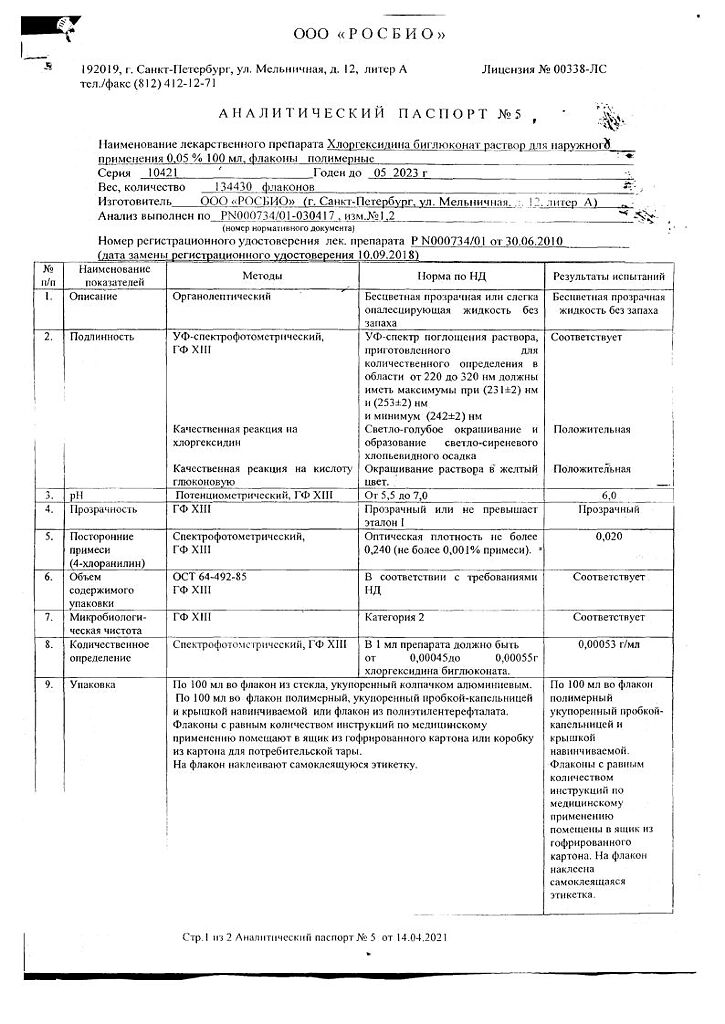
Composition
Composition
Active ingredient:
Chlorhexidine, substance-solution [equivalent to 0.5 g chlorhexidine bigluconate] Excipient:
Purified water
Pregnancy
Pregnancy
During pregnancy and breastfeeding, it is used only if the expected benefit to the mother outweighs the potential risk to the fetus or child.
Before using the drug, if you are pregnant, or think you might be pregnant, or are planning a pregnancy, you should consult your doctor during breastfeeding.
Contraindications
Contraindications
Hypersensitivity, dermatitis, allergic reactions.
With caution
Children’s age, pregnancy, breastfeeding period.
Side Effects
Side Effects
Severe allergic reactions, including anaphylaxis, dry and itchy skin, photosensitivity. In the treatment of oral diseases – staining of tooth enamel, tartar deposits, taste disturbance.
If you experience side effects listed in the instructions or they get worse, or you notice any other effects not listed in the instructions, tell your doctor
Interaction
Interaction
Pharmaceutically incompatible with soap, alkalis and other anionic compounds.
Ethanol enhances the effectiveness of the drug.
Concomitant use with iodine is not recommended.
If you are using the above or other medications (including over-the-counter medications), consult your doctor before using the drug.
Overdose
Overdose
When used externally, overdose is unknown.
Storage conditions
Storage conditions
In a place protected from light at a temperature of 2 to 25 0C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Rosbio, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a cool, protected from light, in a tightly sealed container. |
| Manufacturer | Rosbio, Russia |
| Medication form | topical solution |
| Brand | Rosbio |
Other forms…
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Buy Chlorhexidine, 0.05% solution 100 ml with delivery to USA, UK, Europe and over 120 other countries.