No products in the cart.
Cernevit, lyophilizate 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
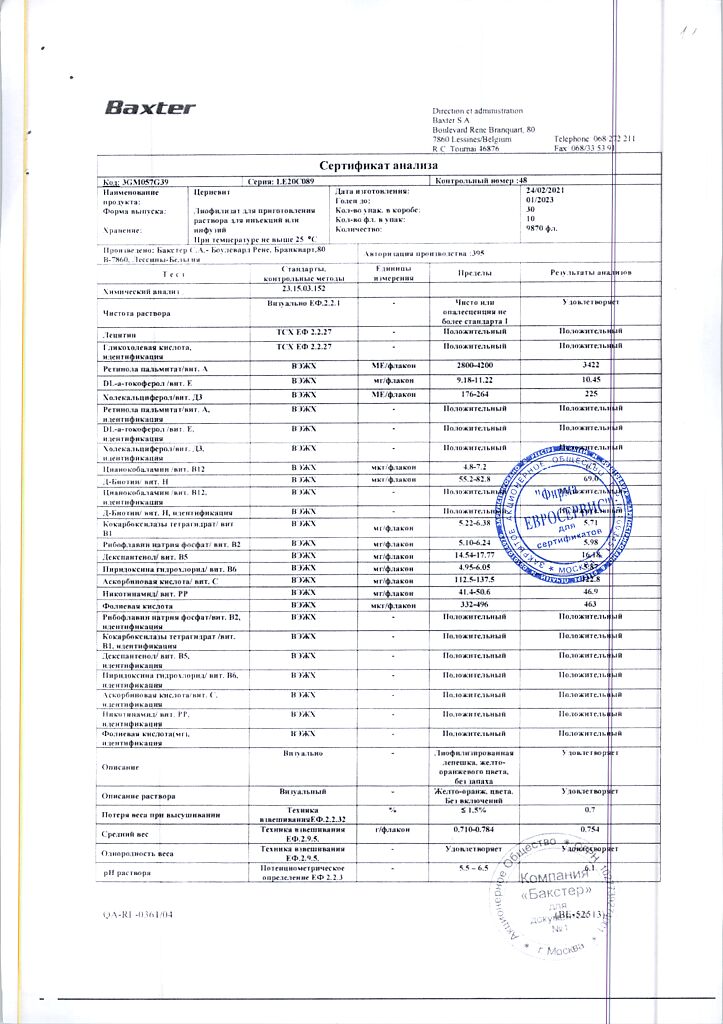
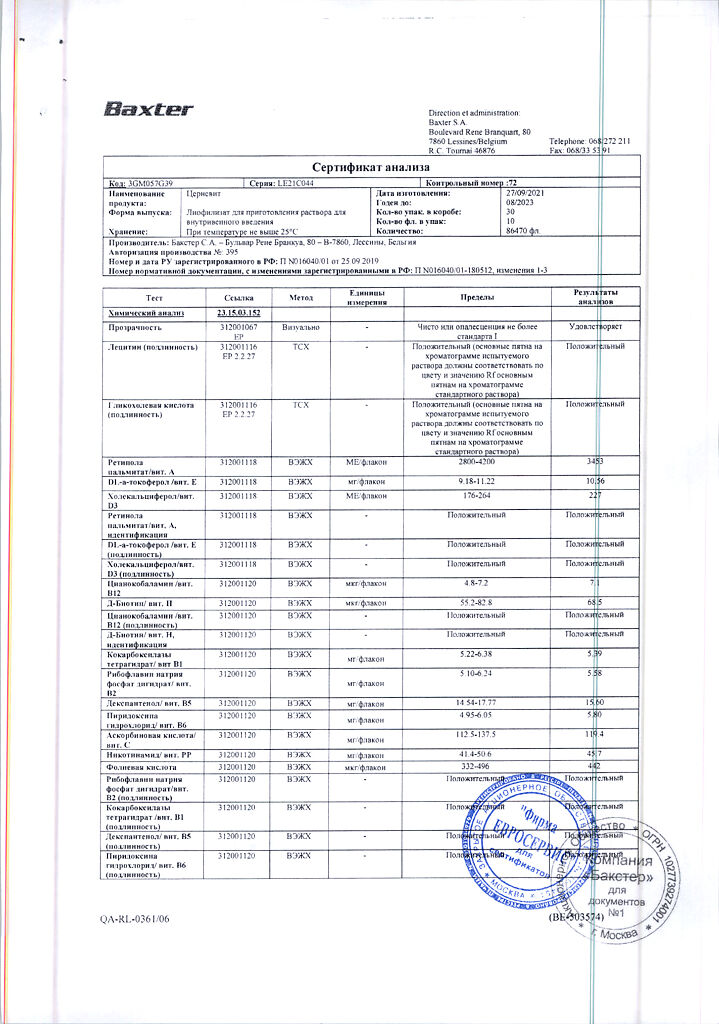
Cernevit is a balanced mixture of water-soluble and fat-soluble vitamins, providing a complete supply of vitamins for parenteral nutrition.
Indications
Indications
Parenteral nutrition supplement for adults and children over 11 years of age.
Composition
Composition
Each vial (5 ml) contains:
Active ingredients:
Retinol (vitamin A) in the form of retinol palmitate 3,500 ME,
Colecalciferol (vitamin D3) 220 ME,
alpha-tocopherol (vitamin E) 11.2 ME, corresponding to the amount of DL alpha-tocopherol 10.2 mg,
Ascorbic acid (vitamin C) 125 mg,
Thiamine (vitamin B1) 3.51mg, in the form of cocarboxylase tetrahydrate 5.8 mg,
Riboflavin (vitamin B2) 4.14 mg, in the form of riboflavin sodium phosphate dihydrate 5.67 mg,
Pyridoxine (vitamin B6) 4.53 mg, in the form of pyridoxine hydrochloride 5.50 mg,
Cyanocobalamin (vitamin B12) 6 µg,
Folic acid (vitamin B9) 414 µg,
Pantothenic acid (vitamin B5) 17.25 mg, in the form of dexpanthenol 16.15 mg,
Biotin (vitamin B8) 0.069 mg,
Nicotinamide (vitamin PP) 46 mg,
Associates:
Glycine,
Glycocholic acid,
Sodium hydroxide,
Hydrocholic acid.
How to take, the dosage
How to take, the dosage
The drug is intended for intravenous administration only.
Dosage:
The recommended dose is 5 ml (1 vial) per day
The method of administration:
Using a syringe, inject 5 ml of water for injection or 5% dextrose (glucose) solution or 0.9% sodium chloride solution into the vial.
Stir gently until the powder is completely dissolved (yellow-orange solution).
The resulting solution is slowly injected intravenously by jetting.
Frequency of administration and duration of therapy:
The use of the drug can be continued for the entire period of parenteral nutrition.
The duration of prescription as recommended by the physician.
Interaction
Interaction
Due to the presence of pyridoxine (vitamin B6) in the drug, co-administration with levodopa preparations is contraindicated.
Due to the presence of folic acid in the drug, caution should be exercised when co-administering with antiepileptic drugs containing phenobarbital, phenytoin or primidone: clinical monitoring and, if possible, monitoring of plasma levels, correction of antiepileptic drug dosing during and after discontinuation of folic acid is necessary.
Compatibility should be checked when preparing with other solutions for infusion, especially if Cernevit is added to binary parenteral mixtures containing glucose, electrolytes and amino acid solutions or to mixtures containing glucose, electrolytes, amino acid solutions and lipids.
Please tell your doctor or pharmacist what medicines you have used before, even if they were prescribed without a prescription.
Special Instructions
Special Instructions
In intravenous bolus administration, rare cases of moderate elevation of ALT (AST) have been reported in patients with active enterocolitis; after discontinuation of Cernevit, elevated “liver” transaminase levels quickly return to normal values. It is recommended to monitor the levels of “hepatic” transaminases in patients of this group.
Because of the presence of glycocholic acid as an adjunct ingredient, during repeated and prolonged administration of the drug in patients with jaundice or pronounced cholestasis (changes in values of laboratory liver function tests) liver function should be carefully monitored.
Deficiency of one or more vitamins should be corrected with special preparations.
Cernevit does not contain vitamin K, which can be taken separately, if necessary.
Contraindications
Contraindications
Overdose
Overdose
Symptoms
Vitamin A overdose of more than 150,000 ME (acute): gastrointestinal disturbances, headache, increased intracranial pressure, optic disc edema, mental disturbances, agitation or even convulsions, delayed generalized epithelial desquamation.
Vitamin A overdose (chronic): increased intracranial pressure, sensitive or painful subcutaneous swellings in the fingers and toes of the upper and lower extremities.
Treatment:
discontinue use of Cernevit, reduce the use of calcium, increase diuresis and conduct adequate rehydration of the body.
No “withdrawal symptoms” after discontinuation of the drug were found.
Pregnancy use
Pregnancy use
Use during pregnancy:
Cernevit may be prescribed during pregnancy, provided the dosing regimen is strictly followed to avoid overdose.
The attending physician or pharmacist should be consulted before using any other drug.
Use during lactation:
The use of Cernevit in breastfeeding women is not recommended because it is possible to develop an overdose of vitamin A in the newborn.
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Pierre Fabre Medical Production, France |
| Medication form | lyophilizate |
| Brand | Pierre Fabre Medical Production |
Related products
Buy Cernevit, lyophilizate 10 pcs with delivery to USA, UK, Europe and over 120 other countries.