No products in the cart.
Description
Pharmacotherapeutic group:
Antitumor drug, estrogen synthesis inhibitor.
The ATC code: L02BG03
Pharmacological properties
Pharmacodynamics
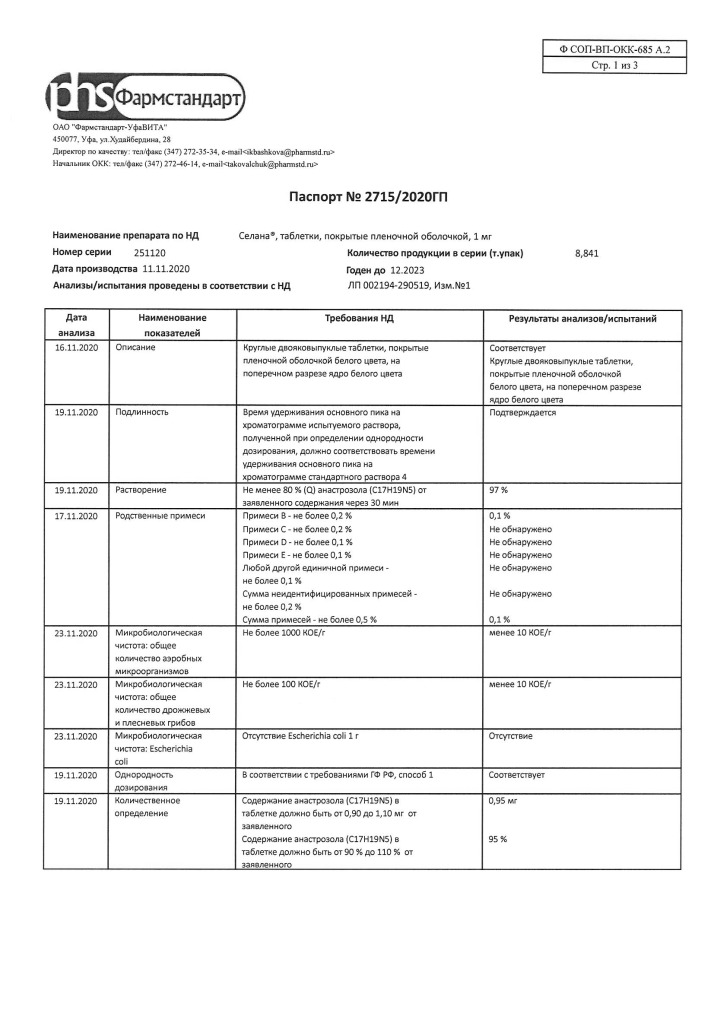
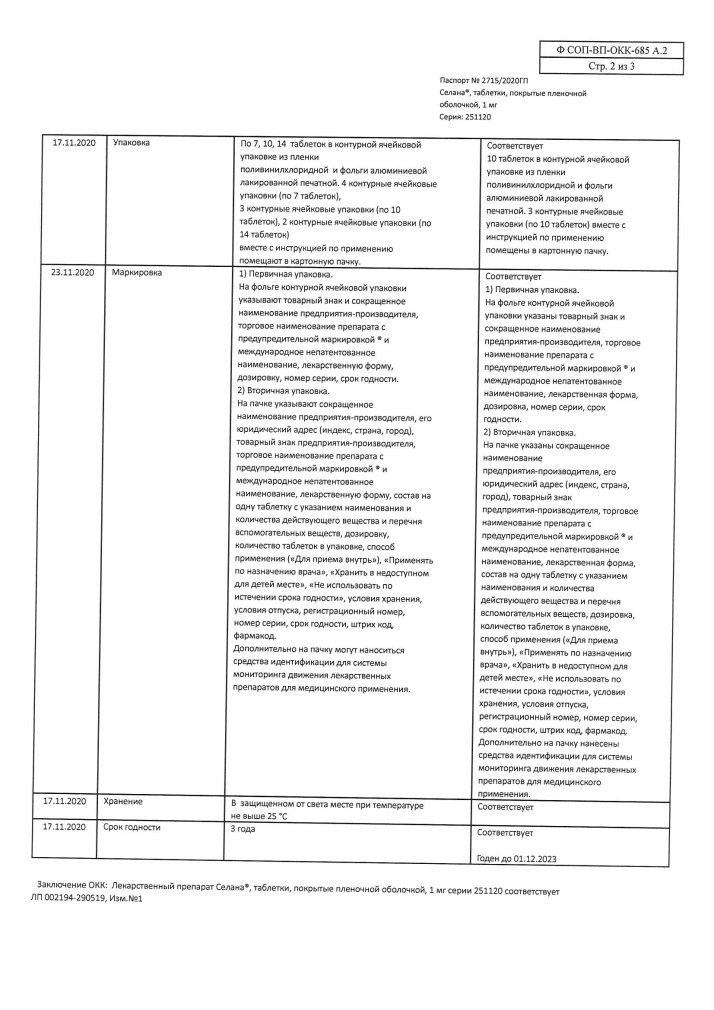
The Anastrozole is a highly selective nonsteroidal aromatase inhibitor. Aromatase is an enzyme by which, in postmenopausal women, androstenedione is converted in peripheral tissues to estrone and then to estradiol. Anastrozole has antitumor activity against estrogen-dependent breast tumors in postmenopausal women. In postmenopausal period the drug in daily dose of 1 mg causes decrease of concentration of estradiol by 80%.
Anastrozole has no progestogenic, androgenic or estrogenic activity.
Anastrozole at a daily dose of up to 10 mg has no effect on cortisol and aldosterone secretion (therefore, no corticosteroid replacement is required with the use of the drug).
Pharmacokinetics
Intake
After oral administration, anastrozole is rapidly absorbed from the gastrointestinal tract. Maximum concentration (Cmax) in plasma is reached within 2 hours (fasting). Food slightly reduces the rate of absorption, but not its degree and does not lead to a clinically significant effect on the equilibrium plasma concentration of the drug in a single daily dose.
Distribution
Anastrozole binds to plasma proteins by 40%. Approximately 90% to 95% of the equilibrium plasma concentration of anastrozole is reached after 7 days of administration. No pharmacokinetic parameters are known to be time and dose dependent.
Metabolism
Anastrozole is metabolized by N-dealkylation, hydroxylation, and glucuronidation. Triazole, the main metabolite determined in plasma, does not inhibit aromatase.
Elimation
Anastrozole is excreted slowly with a half-life (T1/2) of 40 to 50 hours.
Anastrozole and its metabolites are excreted mainly in the urine. Less than 10% of the dose is excreted unchanged in the urine within 72 hours after drug administration. Pharmacokinetics in Special Clinical Cases
The determined clearance of anastrozole after oral administration in patients with cirrhosis or impaired renal function does not differ from that determined in healthy subjects.
The pharmacokinetics of anastrozole are independent of age in postmenopausal women.
Indications
Indications
Adjuvant therapy for early hormone-positive breast cancer in postmenopausal women.
Treatment of advanced breast cancer in postmenopausal women.
Adjuvant therapy for early hormone-positive breast cancer in postmenopausal women after tamoxifen therapy for 2–3 years
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Antitumor agent, estrogen synthesis inhibitor.
ATX code: L02BG03
Pharmacological properties
Pharmacodynamics
Anastrozole is a highly selective non-steroidal aromatase inhibitor. Aromatase is an enzyme that converts androstenedione in peripheral tissues into estrone and then into estradiol in postmenopausal women. Anastrozole has antitumor activity against estrogen-dependent breast tumors in postmenopausal women. In the postmenopausal period, the drug in a daily dose of 1 mg causes a decrease in estradiol concentration by 80%.
Anastrozole does not have progestogenic, androgenic and estrogenic activity.
Anastrozole in a daily dose of up to 10 mg has no effect on the secretion of cortisol and aldosterone (therefore, corticosteroid replacement is not required when using the drug).
Pharmacokinetics
Suction
After oral administration, anastrozole is rapidly absorbed from the gastrointestinal tract. The maximum concentration (Cmax) in plasma is achieved within 2 hours (on an empty stomach). Food slightly reduces the rate of absorption, but not its extent, and does not lead to a clinically significant effect on the steady-state plasma concentration of the drug after a single daily dose.
Distribution
Anastrozole is 40% bound to plasma proteins. After 7 days of taking the drug, approximately 90–95% of the equilibrium concentration of anastrozole in plasma is achieved. There is no information on the dependence of pharmacokinetic parameters on time and dose.
Metabolism
Anastrozole is metabolized by N-dealkylation, hydroxylation and glucuronidation. Triazole, the main metabolite detected in plasma, does not inhibit aromatase.
Removal
Anastrozole is excreted slowly, the half-life (T1/2) is 40 – 50 hours.
Anastrozole and its metabolites are excreted primarily in the urine. Less than 10% of the dose is excreted unchanged in the urine within 72 hours after taking the drug. Pharmacokinetics in special clinical situations
The determined clearance of anastrozole after oral administration in patients with liver cirrhosis or impaired renal function does not differ from the clearance determined in healthy subjects.
The pharmacokinetics of anastrozole does not depend on age in postmenopausal women.
Special instructions
Special instructions
The patient’s menopausal hormonal status should be confirmed by determining sex hormones in the blood serum.
The effectiveness of anastrozole in women with estrogen receptor-negative tumors has not been noted, unless there has been a previous positive clinical response to tamoxifen.
There are no data on the safety of anastrozole in patients with severe liver dysfunction or in patients with severe renal failure (creatinine clearance less than 20 ml/min).
In case of persistent uterine bleeding while taking anastrozole, consultation and observation of a gynecologist is necessary.
Drugs containing estrogens should not be prescribed concomitantly with anastrozole. By reducing the concentration of circulating estradiol, anastrozole can cause a decrease in bone mineral density.
In patients with osteoporosis or at risk of developing osteoporosis, bone mineral density should be assessed by densitometry (for example, DEXA scanning) at the beginning of treatment and over time. If necessary, treatment or prevention of osteoporosis should be started under close medical supervision. There is no data on the simultaneous use of anastrozole and gonadotropin-releasing hormone (GnRH) analogues. It is not known whether anastrozole improves treatment outcomes when used concomitantly with chemotherapy.
Safety data for long-term treatment with anastrozole have not yet been obtained.
The effectiveness and safety of anastrozole and tamoxifen when used simultaneously, regardless of hormonal receptor status, are comparable to those when using tamoxifen alone. The exact mechanism of this phenomenon is not yet known.
Impact on the ability to drive vehicles and machinery
Care should be taken when driving vehicles and machinery, because some side effects of the drug, such as asthenia, headache, dizziness and sleep disturbances, may negatively affect the ability to perform work that requires increased concentration and speed of psychomotor reactions. If these side effects occur, you should refrain from performing these activities.
Active ingredient
Active ingredient
Anastrozole
Composition
Composition
(1 table):
active substance:
anastrozole 1.0 mg;
excipients:
magnesium aluminum metasilicate 9.0 mg,
croscarmellose sodium 3.0 mg,
colloidal silicon dioxide 3.0 mg,
magnesium stearate 0.5 mg,
ludipress in terms of components 83.5 mg (lactose monohydrate 77.7 mg, povidone 2.9 mg, crospovidone 2.9 mg).
Shell:
Opadry II white 85F48105 (polyvinyl alcohol from 35.0 to 49.00%, talc from 9.80 to 25.00%, macrogol 3350 from 7.35 to 35.20%, titanium dioxide from 15.15 to 30.00%)
Contraindications
Contraindications
Hypersensitivity to anastrozole and other components of the drug.
Severe renal failure (creatinine clearance less than 20 ml/min).
Severe liver failure (safety and effectiveness have not been established).
Concomitant therapy with tamoxifen or drugs containing estrogens.
Children’s age (safety and effectiveness have not been established).
Premenopausal period.
Pregnancy and breastfeeding period.
With caution
Osteoporosis, hypercholesterolemia, lactase deficiency, lactose intolerance, glucose-galactose malabsorption (the dosage form of the drug contains lactose), coronary heart disease, liver dysfunction.
Side Effects
Side Effects
Side effects recorded more often than single observations are listed below by organ and system, indicating the frequency of their occurrence.
Determination of the frequency of adverse reactions: very often (> 10%); often (1-10%); infrequently (0.1 – 1%); rarely (0.01 – 0.1%); very rare (<0.01%).
From the cardiovascular system: very often – hot flashes, increased blood pressure; often – vasodilation, ischemic cardiovascular diseases.
From the musculoskeletal system: very often – arthralgia, joint stiffness, arthritis, back pain, decreased bone mineral density, osteoporosis and bone fractures; often – bone pain, myalgia; infrequently – “trigger finger” or “snapping finger” (impaired ability to straighten the finger, usually the middle or ring finger).
From the reproductive and genitourinary system: often – dryness of the vaginal mucosa, vaginal bleeding (mainly during the first weeks after discontinuation or change of previous hormonal therapy to anastrozole drugs), vulvovaginitis, vaginitis, pelvic pain; uncommon – mucous vaginal discharge, urinary tract infections, chest pain; rarely – endometrial cancer, breast tumors.
From the blood and lymphatic system: very often – lymphedema; infrequently – anemia.
From the digestive system: very often – nausea, vomiting; often – diarrhea, constipation, dyspepsia, increased activity of alkaline phosphatase, alania aminotransferase, aspartate aminotransferase; uncommon – abdominal pain, increased gamma-glutamine transferase activity and bilirubin concentration, hepatitis, dry mouth.
From the nervous system: very often – headache, dizziness, sleep disturbances, depression; often – carpal tunnel syndrome (mainly observed in patients with risk factors for this disease), anxiety, paresthesia.
From the senses: infrequently – cataracts.
From the respiratory system: very often – pharyngitis, increased cough, shortness of breath; uncommon – chest pain, sinusitis, bronchitis.
Metabolism: very often – peripheral edema; often – anorexia, hypercholesterolemia, hypercalcemia (with or without increased parathyroid hormone concentration), weight gain.
From the skin and appendages: very often – skin rash; often – thinning hair, alopecia; uncommon – erythema multiforme (Stevens-Johnson syndrome), cutaneous vasculitis (including isolated cases of purpura (Schönlein-Henoch syndrome)).
Allergic reactions: often – allergic reactions; uncommon – urticaria; rarely – anaphylactoid reaction; very rarely – angioedema.
Other: very often – asthenia.
If you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
Drug interactions with phenazone (Antipyrine) and cimetidine indicate that coadministration of anastrozole with other drugs is unlikely to result in a clinically significant interaction due to cytochrome P450.
There are no data on clinically significant drug interactions when taking anastrozole concomitantly with other commonly used drugs.
At the moment, there is no information on the use of anastrozole in combination with other anticancer drugs.
Preparations containing estrogens should not be used simultaneously with anastrozole, because they reduce the pharmacological effect of the latter.
Tamoxifen should not be used concomitantly with anastrozole, as it may weaken the pharmacological effect of the latter.
Overdose
Overdose
Isolated clinical cases of anastrozole overdose have been described. The single dose of anastrozole at which life-threatening symptoms develop has not been established.
Treatment: There is no specific antidote. If necessary, carry out symptomatic therapy. Initiate vomiting (if the patient is conscious), conduct general supportive therapy and monitor the patient, monitor the functions of vital organs and systems. Dialysis possible
Manufacturer
Manufacturer
Pharmstandard-UfaVITA, Russia
Additional information
| Manufacturer | Pharmstandard-UfaVITA, Russia |
|---|---|
| Medication form | pills |
| Brand | Pharmstandard-UfaVITA |
Related products
Buy Celana, 1 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.