No products in the cart.
Ceftazidime, 1 g
€1.00
Out of stock
(E-mail when Stock is available)
Description
The drug Ceftazidime is an antibacterial drug of III generation cephalosporins group, has a broad spectrum and bactericidal effect, disrupts cell wall synthesis of microorganisms, resistant to the action of most beta-lactamases. The drug is active against Gram-negative microorganisms: Haemophilus influenzae, Neisseria gonorrhoeae and other Neisseria spp.
Indications
Indications
Infectious and inflammatory diseases caused by microorganisms sensitive to the drug:
Active ingredient
Active ingredient
Composition
Composition
Active substance:
1 g of ceftazidime pentahydrate sodium carbonate (in terms of ceftazidime).
How to take, the dosage
How to take, the dosage
The drug Ceftazidime-ACOS is used only parenterally: intravenously (by stream or dropwise) or intramuscularly. The dose of the drug is set individually, taking into account the severity of the disease, localization of infection, type and sensitivity of the pathogen, age and renal function.
The drug is administered intravenously (by trickle or drop) or deeply intravenously in the area of the upper outer quadrant of the greater gluteal muscle or in the area of the lateral thigh. Ceftazidime solution may be injected directly into a vein or into a tube of an infusion system.
The usual dose for adults and children over 12 years of age is 1 g in m/m or v/v every 8-12 hours.
In uncomplicated urinary tract infections, 250 mg every 12 hours in an intravenous or intravenous route.
In complicated urinary tract infections, 0.5-1 g every 8-12 hrs in an oral or intravenous mode.
In case of uncomplicated pneumonia, skin and soft tissue infections – intravenous or intravenous 0.5-1 g every 8 hours.
In case of cystic fibrosis, infections of the respiratory tract caused by Pseudomonas spp.-into a dose of 100-150 mg/kg/day, the frequency of administration is 3 times/day (using a dose of up to 9 g/day in these patients had no complications).
Infections of bones and joints – IV 2 g every 12 hours.
In severe infections, including nosocomial infections, 2 g every 8 hours.
In extremely severe or life-threatening infections – IV 2 g every 8 hours.
In elderly patients the maximum daily dose should not exceed 3 g.
Patients with renal insufficiency require dose reduction because ceftazidime is excreted unchanged by the kidneys. The initial dose is 1 g. The maintenance dose is adjusted depending on glomerular filtration rate.
Patients with severe infections may increase the maintenance dose by 50% or increase the frequency of administration. In this case, serum ceftazidime levels should be monitored; serum ceftazidime concentration should not exceed 40 mg/L.
In children, creatinine clearance is calculated according to ideal weight or body surface area.
The T1/2 drug during hemodialysis is 3-5 h. The appropriate dose of the drug should be repeated after each period of dialysis.
In peritoneal dialysis, ceftazidime may be included in the dialysis solution at a dose of 125 mg to 250 mg per 2 L of dialysis solution.
In patients with renal failure who are on continuous hemodialysis using an arteriovenous shunt and in patients on high rate hemofiltration in the intensive care unit, the recommended doses are 1 g/day daily (in 1 or more administrations).
In patients on low-rate hemofiltration, doses recommended for impaired renal function are administered.
In children under 2 months of age, 25-60 mg/kg/day (in 2 injections) is prescribed.
Children from 2 months to 12 years of age are prescribed 30-100 mg/kg/day (in 2-3 injections).
In children with reduced immunity, cystic fibrosis and meningitis, 150 mg/kg/day (in 3 injections).
The maximum daily dose of ceftazidime for children is 6 g.
The duration of treatment
The duration of treatment with ceftazidime is 7-14 days. In infections caused by Pseudomonas aeruginosa (pneumonia, cystic fibrosis, meningitis) the course of treatment may be increased up to 21 days.
Regulations for preparing solutions
Carbon dioxide (carbon dioxide) is released when the powder is dissolved. After the solvent is injected, the bottle should be shaken to produce a clear solution. In the resulting ready solution of the drug there may be small bubbles of carbon dioxide (carbon dioxide).
The resulting solution may be light yellow to dark yellow. If all the recommended dilution rules are followed, the effectiveness of the product is not affected by the shade.
Primary dilution
Secondary dilution
For IV (intravenous) drip administration the solution of Ceftazidime-ACOS obtained in the above described manner is additionally diluted in 50-100 ml of one of the following solvents intended for IV administration (0.9% sodium chloride solution, Ringer’s solution, 5% or 10% dextrose (glucose) solution, 5% dextrose (glucose) solution with 0.9% sodium chloride solution. For secondary dilution, only freshly prepared solution should be used.
Interaction
Interaction
Antibacterial synergism is noted when concomitant use with aminoglycosides.
“Loop” diuretics, aminoglycosides, vancomycin, clindamycin reduce clearance of ceftazidime, resulting in an increased risk of nephrotoxic effect.
The bacteriostatic antibiotics (including chloramphenicol) reduce the effect of the drug.
Pharmaceutical interactions
Do not use sodium hydrocarbonate solution as a solvent (carbon dioxide is formed, this may require gas release to the outside).
Pharmaceutically incompatible with aminoglycosides (significant mutual inactivation: these drugs should be injected in different parts of the body if used simultaneously) and vancomycin (forms a precipitate depending on the concentration; if two drugs must be injected through the same tube, IV systems should be flushed between applications).
Pharmaceutically compatible with the following solutions: at concentrations from 1 to 40 mg/ml – sodium chloride 0.9%, sodium lactate, Hartmann’s solution, dextrose (glucose) 5%, sodium chloride 0.225% and dextrose (glucose) 5%, sodium chloride 0.45% and dextrose (glucose) 5%, sodium chloride 0.9% and dextrose (glucose) 5%, sodium chloride 0.18% and dextrose (glucose) 4%, dextrose (glucose) 10%, dextran 40 (10%) in sodium chloride solution 0.9%, dextran 40 (10%) in dextrose (glucose) solution 5%, dextran 70 (6%) in sodium chloride solution 0.9%, dextran 70 (6%) in dextrose (glucose) solution 5%.
At concentrations of 0.05 to 0.25 mg/ml ceftazim is compatible with intraperitoneal dialysis solution (lactate).
For intravenous administration, ceftazidime may be diluted with 0.5% or 1% lidocaine hydrochloride solution. Both components remain active if ceftazidime is added to the following solutions (ceftazidime concentration 4 mg/ml): hydrocortisone, (hydrocortisone sodium phosphate) 1 mg/ml in sodium chloride 0.9% or 5% dextrose (glucose) solution, cefuroxime (cefuroxime sodium) 3 mg/ml in 0.9% sodium chloride solution, cloxacillin (cloxacillin sodium) 4 mg/ml in 0.9% sodium chloride solution, heparin 10 IU/ml in 0.9% sodium chloride solution, potassium chloride 10 mEq/L or 40 mEq/L in 0.9% sodium chloride solution.
When mixing a solution of ceftazidime (500 mg in 1.5 ml of water for injection) and metronidazole (500 mg/100 ml) both components retain their activity.
Special Instructions
Special Instructions
Patients with a history of allergic reactions to penicillins may have hypersensitivity to cephalosporin antibiotics.
Patients with a history of allergic reactions to penicillin may be hypersensitive to cephalosporin antibiotics.
When using the drug and 2-3 weeks after discontinuation of treatment, development of diarrhea caused by Clostridium difficile is possible. In mild cases withdrawal of treatment and use of ion exchange resins (colestyramine, colestipol) is sufficient, in severe cases compensation of loss of fluid, electrolytes and protein, prescription of vancomycin, bacitracin or metronidazole is indicated. Do not use drugs that inhibit intestinal peristalsis.
Impact on driving and operating machinery
At the time of treatment, caution should be exercised while driving vehicles and engaging in other potentially dangerous activities requiring increased concentration and quick psychomotor reactions because of the risk of dizziness.
Contraindications
Contraindications
With caution: renal failure, colitis in anamnesis, malabsorption syndrome (increased risk of decreased prothrombin activity, especially in persons with severe renal and/or hepatic failure), simultaneous administration with a “loop” diuretic and aminoglycoside, and in newborns.
Side effects
Side effects
Allergic reactions: urticaria, chills or fever, rash, itching, bronchospasm, eosinophilia, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome), angioedema, anaphylactic shock.
Digestive system disorders: nausea, vomiting, diarrhea, flatulence, abdominal pain, dysbacteriosis, increased liver transaminase activity, alkaline phosphatase, hyperbilirubinemia, stomatitis, glossitis, pseudomembranous enterocolitis, oropharyngeal candidiasis, cholestasis.
Hematopoietic system disorders: eosinophilia, leukopenia, neutropenia, agranulocytosis, granulocytopenia, thrombocytopenia, hemolytic anemia, lymphocytosis, hemorrhages.
Perior genital system disorders: candidal vaginitis.
Urinary system disorders: renal dysfunction, toxic nephropathy.
CNS disorders: headache, dizziness, paresthesias, dizziness, seizures, encephalopathy, “fluttering tremor”.
Laboratory parameters: hypercreatininemia, increased concentration of urea, false positive urine glucose reaction, false positive direct Coombs reaction, increased prothrombin time.
Local reactions: when administered intravenously – phlebitis; when administered intramuscularly – pain, burning, thickening at the injection site.
Other: nasal bleeding, superinfection.
Overdose
Overdose
Symptoms: pain, inflammation, phlebitis at the injection site, dizziness, paresthesias, headache, seizures in patients with renal failure, hypercreatininemia, hyperbilirubinemia, thrombocytosis, thrombocytopenia, eosinophilia, leukopenia, prolonged prothrombin time.
Treatment: symptomatic therapy; in case of renal failure – peritoneal dialysis or hemodialysis.
Similarities
Similarities
Additional information
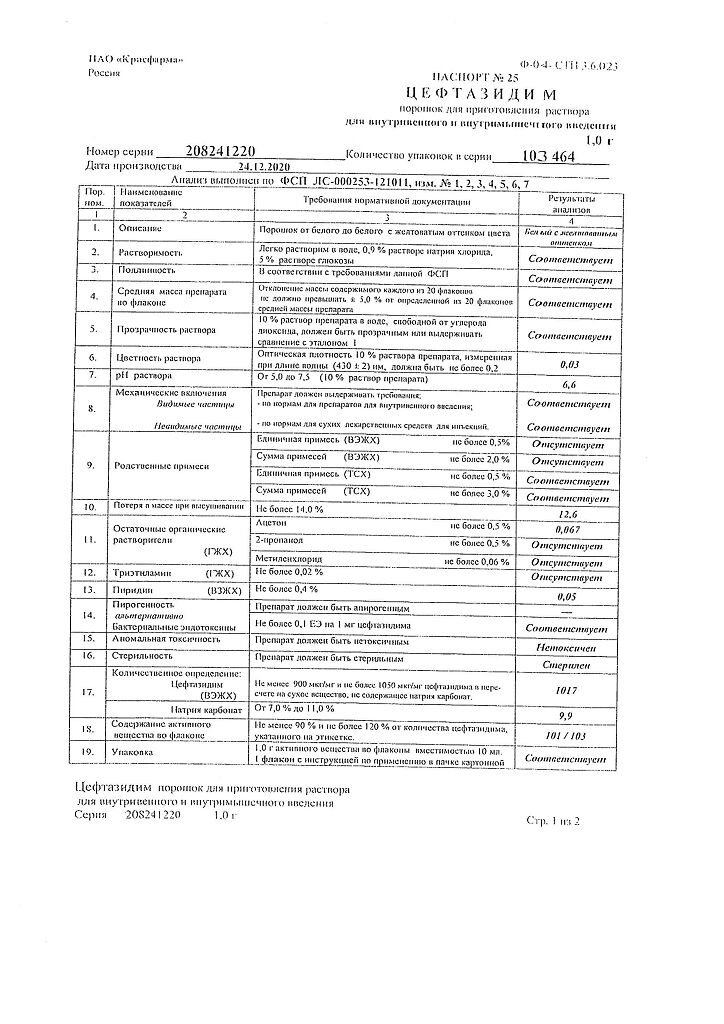
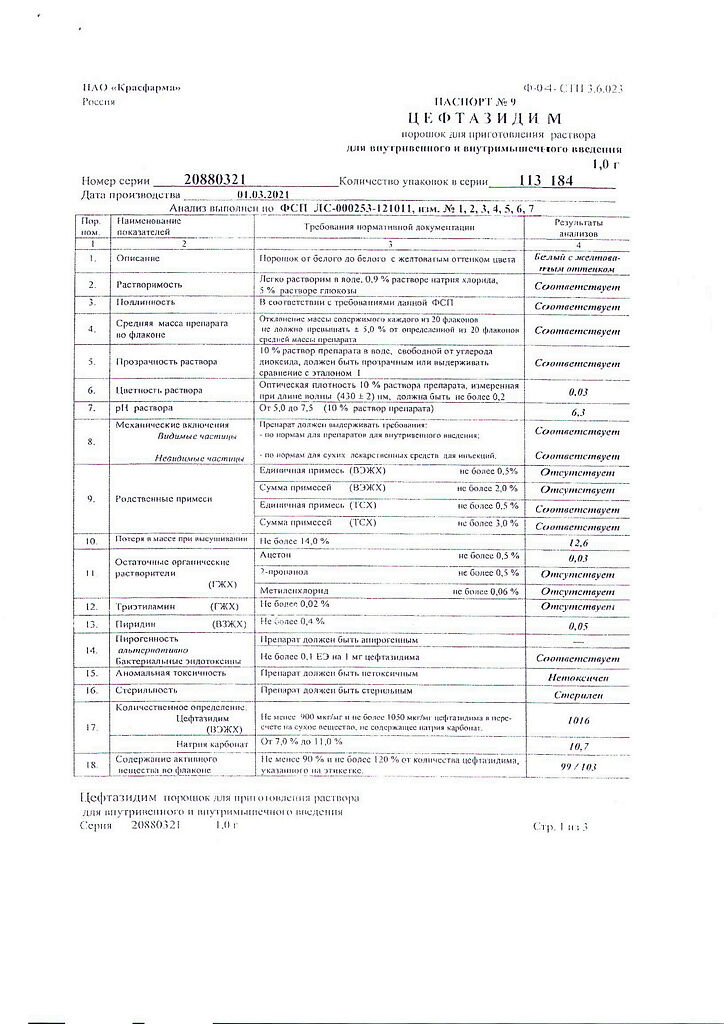
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
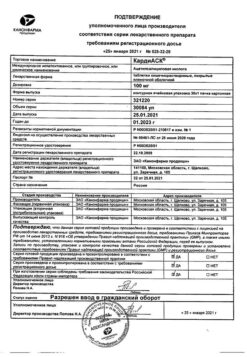
| Manufacturer | Kraspharma PJSC, Russia |
| Medication form | Powder for preparation of solution |
| Brand | Kraspharma PJSC |
Other forms…
Related products
Buy Ceftazidime, 1 g with delivery to USA, UK, Europe and over 120 other countries.