No products in the cart.
Cefepim, 1 g
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group:
Cephalosporin antibiotic
ATX code:
J01DE01
Pharmacological properties
Pharmacodynamics
Cefepim is a broad spectrum cephalosporin antibiotic. Cefepime inhibits the protein synthesis of the bacterial cell wall and has a broad spectrum of bactericidal action against various Gram-positive and Gram-negative bacteria, including most strains resistant to aminoglycosides or third generation cephalosporin antibiotics, such as ceftazidime. Cefepime is highly resistant to hydrolysis by most beta-lactamases, it has low affinity for beta-lactamases and penetrates rapidly into Gram-negative bacteria cells. Cefepime has been shown to have a very high affinity for penicillin-binding protein (PBP) type 3, a high affinity for PBP type 2 and a moderate affinity for PBP types 1a and 1b. Cefepime has bactericidal activity against a wide range of bacteria. Cefepim is active against the following microorganisms:
Gram-positive aerobes:
Staphylococcus aureus (including beta-lactamase-producing strains);
Staphylococcus epidermidis (including beta-lactamase-producing strains);
other strains of Staphylococcus spp, including Staphylococcus hominis, Staphylococcus saprophyticus;
Streptococcus pyogenes (group A streptococci);
Streptococcus agalactiae (group B streptococci);
Streptococcus pneumoniae (including strains with medium resistance to penicillin – minimum suppressive concentration from 0.1 to 1 mcg/ml);
Other beta-haemolytic Streptococcus spp. (groups C, G, F), Streptococcus bovis (group D), Streptococcus spp. group viridans.
Note: Most strains of enterocococci, such as Enterococcus faecalis, and staphylococci resistant to methicillin are resistant to most cephalosporin antibiotics, including cefepime.
Gram-negative aerobes:
Acinetobacter calcoaceticus (sub strains anitratus, Iwoffii);
Aeromonas hydrophila;
Capnocytophaga spp;
Citrobacter spp, including Citrobacter diversus, Citrobacter freundii,
Campylobacter jejuni;
Enterobacter spp, Including Enterobacter cloacae, Enterobacter aerogenes, Enterobacter sakazakii;
Escherichia coli;
Gardnerella vaginalis;
Haemophilus ducreyi;
Haemophilus influenzae (including beta-lactamase-producing strains);
Haemophilus parainfluenzae;
Hafnia alvei;
Klebsiella spp., including Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella ozaenae;
Legionella spp.
Morganella morganii;
Moraxella catarrhalis (Branhamella catarrhalis) (including strains producing beta-lactamases);
Neisseria gonorrhoeae (including strains producing beta-lactamase);
Neisseria meningitidis;
Pantoea agglomerans (previously known as Enterobacter agglomerans);
Proteus spp., including Proteus mirabilis, Proteus vulgaris;
Providencia spp, including Providencia rettgeri, Providencia stuartii;
Pseudomonas spp, including Pseudomonas aeruginosa, Pseudomonas putida, Pseudomonas stutzeri, Salmonella spp.;
Serratia, including Serratia marcescens, Serratia liquefaciens;
Shigella spp.;
Yersinia enterocolitica.
Note: cefepim is inactive against many strains of Stenotrophomonas maltophilia (previously known as Xanthomonas maltophilia and Pseudomonas maltophilia).
Anaerobes:
Bacteroides spp;
Clostridium perfringens;
Fusobacterium spp;
Mobiluncus spp;
Peptostreptococcus spp.
Prevotella melaninogenica (known as Bacteroides melaninogenicus);
Veillonella spp.
Note: cefepime is inactive against Bacteroides fragilis and Clostridium difficile.
Pharmacokinetics
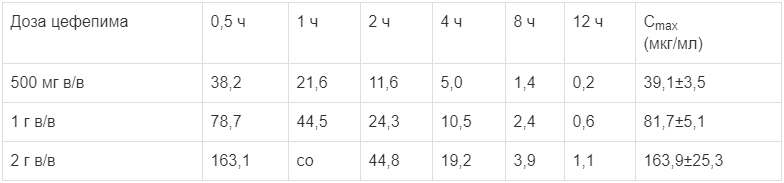
Mean plasma concentrations of cefepime in healthy adult men at different times after a single intravenous injection for 30 minutes to 12 hours and the maximum concentration (Cmax) are shown in the table below.
The mean plasma concentrations of cefepime (µg/ml) after intravenous administration

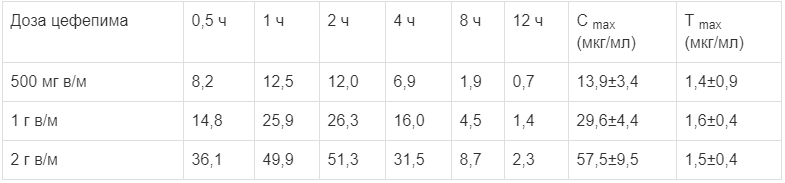
The cmax and time to maximum concentration (Tmax) after a single intramuscular injection are shown in the table below.
The mean plasma concentrations of cefepime (µg/ml) after intramuscular administration

Cefepime is metabolized to N-methylpyrrolidine, which is rapidly converted to N-methylpyrrolidine oxide.
Cefepim is excreted mainly by the kidneys, by glomerular filtration (renal clearance averages 110 ml/min). About 85% of the administered dose of unchanged cefepime, less than 1% of N-methylpyrrolidine, about 6.8% of N-methylpyrrolidine oxide and about 2.5% of cefepime epimer are found in the urine.
After administration of doses from 250 mg to 2 g the half-life of cefepime from the body is on average about 2 hours. Total clearance averages 120 ml/min. No cumulation of the drug was observed when administered intravenously to healthy volunteers at a dose of 2 g every 8 hours for 9 days.
Patients with impaired renal function
The elimination half-life in renal failure is prolonged, with a linear relationship between total clearance and creatinine clearance. In severe renal function disorders that require dialysis sessions, the half-life is on average 13 hours in hemodialysis and 19 hours in continuous peritoneal dialysis. Dose adjustment is required if renal function is impaired.
Patients with impaired hepatic function
Pharmacokinetics of cefepime in patients with impaired hepatic function does not change. No dose adjustment is required for these patients.
Patients older than 65 years
After a single intravenous infusion of 1 g of the drug in healthy volunteers older than 65 years, an increase in the area under the curve “concentration-time” (AUC) and decrease in renal clearance as compared to young volunteers were noted. Dose adjustment is required in elderly patients with impaired renal function.
In children
Pharmacokinetics of the drug was studied in children aged 2 months to 11 years after a single dose of 50 mg/kg body weight intravenously or intramuscularly as well as after repeated administration of the drug (every 8-12 hours, for at least 48 hours). After a single intravenous injection, total clearance and volume of distribution were 3.3 ml/min/kg and 0.3 l/kg, respectively. The elimination half-life averaged 1.7 h. Renal excretion of cefepime unchanged was 60.4% of the administered dose, and renal clearance averaged 2.0 ml/min/kg.
After multiple intravenous administration, equilibrium plasma cefepime concentrations and other pharmacokinetic parameters did not differ from those after single administration. Patients’ age and sex had no significant effect on total clearance and volume of distribution adjusted for body weight. After intramuscular administration the maximum plasma concentration of cefepime in equilibrium averaged 68 µg/ml and was reached on average within 0.75 h. Eight hours after intramuscular administration plasma concentrations of cefepime averaged 6 µg/ml. Absolute bioavailability of cefepime after intramuscular injection averaged 82%.
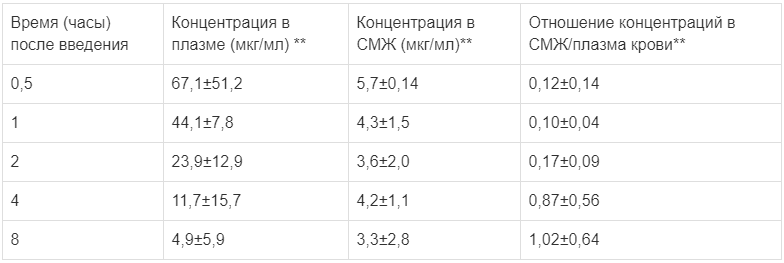
The drug concentrations in cerebrospinal fluid (CSF) and plasma in children with bacterial meningitis

Indications
Indications
Infectious and inflammatory diseases caused by microorganisms sensitive to cefepime in adults:
– Lower respiratory tract infections, including pneumonia and bronchitis;
– Urinary tract infections, both complicated, including pyelonephritis, and uncomplicated;
– Skin and soft tissue infections;
– Abdominal infections, including peritonitis and biliary tract infections;
– Gynecological infections;
– Septicemia;
– Febrile neutropenia.
Prevention of possible infections during abdominal surgery.
Infectious and inflammatory diseases caused by microorganisms sensitive to cefepime in children:
– Pneumonia;
– Urinary tract infections, both complicated, including pyelonephritis, and uncomplicated;
– Skin and soft tissue infections;
– Septicemia;
– Febrile neutropenia;
– Bacterial meningitis.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
antibiotic-cephalosporin
ATX Code:
J01DE01
Pharmacological properties
Pharmacodynamics
Cefepime is a broad-spectrum cephalosporin antibiotic. Cefepime inhibits the synthesis of bacterial cell wall proteins and has a wide spectrum of bactericidal action against various gram-positive and gram-negative bacteria, including most strains resistant to aminoglycosides or third-generation cephalosporin antibiotics such as ceftazidime. Cefepime is highly resistant to hydrolysis by most beta-lactamases, it has low affinity for beta-lactamases and quickly penetrates into the cells of gram-negative bacteria. Cefepime has been shown to have a very high affinity for penicillin binding protein (PBP) type 3, a high affinity for PBP type 2 and a moderate affinity for PBP types 1a and 1b. Cefepime has a bactericidal effect against a wide range of bacteria. Cefepime is active against the following microorganisms:
Gram-positive aerobes:
Staphylococcus aureus (including beta-lactamase producing strains);
Staphylococcus epidermidis (including beta-lactamase producing strains);
other strains of Staphylococcus spp., including Staphylococcus hominis, Staphylococcus saprophyticus;
Streptococcus pyogenes (group A streptococci);
Streptococcus agalactiae (group B streptococci);
Streptococcus pneumoniae (including strains with intermediate resistance to penicillin – minimum inhibitory concentration of 0.1 to 1 μg/ml);
other beta-hemolytic Streptococcus spp. (groups C, G, F), Streptococcus bovis (group D), Streptococcus spp. viridans groups.
Note: Most strains of enterococci, such as Enterococcus faecalis, and methicillin-resistant staphylococci are resistant to most cephalosporin antibiotics, including cefepime.
Gram-negative aerobes:
Acinetobacter calcoaceticus (substrains anitratus, Iwoffii);
Aeromonas hydrophila;
Capnocytophaga spp.;
Citrobacter spp., including Citrobacter diversus, Citrobacter freundii,
Campylobaster jejuni;
Enterobacter spp., including Enterobacter cloacae, Enterobacter aerogenes, Enterobacter sakazakii;
Escherichia coli;
Gardnerella vaginalis;
Haemophilus ducreyi;
Haemophilus influenzae (including beta-lactamase producing strains);
Haemophilus parainfluenzae;
Hafnia alvei;
Klebsiella spp., including Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella ozaenae;
Legionella spp.;
Morganella morganii;
Moraxella catarrhalis (Branhamella catarrhalis) (including beta-lactamase producing strains);
Neisseria gonorrhoeae (including beta-lactamase producing strains);
Neisseria meningitidis;
Pantoea agglomerans (formerly known as Enterobacter agglomerans);
Proteus spp., including Proteus mirabilis, Proteus vulgaris;
Providencia spp., including Providencia rettgeri, Providencia stuartii;
Pseudomonas spp., including Pseudomonas aeruginosa, Pseudomonas putida, Pseudomonas stutzeri, Salmonella spp.;
Serratia, including Serratia marcescens, Serratia liquefaciens;
Shigella spp.;
Yersinia enterocolitica.
Note: Cefepime is inactive against many strains of Stenotrophomonas maltophilia (formerly known as Xanthomonas maltophilia and Pseudomonas maltophilia).
Anaerobes:
Bacteroides spp.;
Clostridium perfringens;
Fusobacterium spp.;
Mobiluncus spp.;
Peptostreptococcus spp.;
Prevotella melaninogenica (known as Bacteroides melaninogenicus);
Veillonella spp.
Note: Cefepime is inactive against Bacteroides fragilis and Clostridium difficile.
Pharmacokinetics
The average concentrations of cefepime in the blood plasma of adult healthy men at various times after a single intravenous administration for 30 minutes to 12 hours and the maximum concentration (Cmax) are shown in the table below.
Mean plasma concentrations of cefepime (µg/ml) after intravenous administration
After intramuscular administration, cefepime is completely absorbed.
Cmax and time to reach maximum concentration (Tmax) after a single intramuscular injection are given in the table below.
Mean plasma concentrations of cefepime (µg/ml) after intramuscular administration
Therapeutic concentrations of cefepime are found in the following fluids and tissues: urine, bile, peritoneal fluid, bullous fluid, bronchial mucosa, sputum, prostate, appendix and gall bladder. The binding of cefepime to serum proteins averages 16.4% and does not depend on the concentration of the drug in the blood serum.
Cefepime is metabolized to N-methylpyrrolidine, which is rapidly converted to N-methylpyrrolidine oxide.
Cefepime is excreted primarily by the kidneys, by glomerular filtration (renal clearance averages 110 ml/min). Approximately 85% of the administered dose of unchanged cefepime, less than 1% N-methylpyrrolidine, about 6.8% N-methylpyrrolidine oxide and about 2.5% cefepime epimer are found in urine.
After administration of doses from 250 mg to 2 g, the half-life of cefepime from the body averages about 2 hours. The total clearance averages 120 ml/min. When the drug was administered intravenously to healthy volunteers at a dose of 2 g every 8 hours for 9 days, no accumulation of the drug was observed.
Patients with impaired renal function
The half-life from the body increases in renal failure, and a linear relationship is observed between total clearance and creatinine clearance. In severe renal impairment requiring dialysis sessions, the half-life averages 13 hours with hemodialysis and 19 hours with continuous peritoneal dialysis. If renal function is impaired, dose adjustment is required.
Patients with liver dysfunction
The pharmacokinetics of cefepime in patients with impaired liver function does not change. No dose adjustment is required for such patients.
Patients over 65 years of age
After a single intravenous administration of 1 g of the drug to healthy volunteers over 65 years of age, an increase in the area under the concentration-time curve (AUC) and a decrease in renal clearance were observed compared with young volunteers. In case of impaired renal function, older patients require dose adjustment.
Children
The pharmacokinetics of the drug was studied in children aged 2 months to 11 years after a single dose of 50 mg/kg body weight intravenously or intramuscularly, as well as after repeated administration of the drug (every 8-12 hours, for at least 48 hours). After a single intravenous dose, total clearance and volume of distribution were 3.3 ml/min/kg and 0.3 L/kg, respectively. The half-life from the body averaged 1.7 hours. Excretion of unchanged cefepime by the kidneys amounted to 60.4% of the administered dose, and renal clearance averaged 2.0 ml/min/kg.
After repeated intravenous administration, the concentration of cefepime in the blood plasma at steady state, as well as other pharmacokinetic parameters, did not differ from those after a single administration. Patient age and gender did not have a significant effect on total clearance and volume of distribution when adjusted for body weight. After intramuscular administration, the maximum concentration of cefepime in the blood plasma at steady state averaged 68 μg/ml and was achieved on average in 0.75 hours. 8 hours after intramuscular administration, the concentration of cefepime in the blood plasma averaged 6 μg/ml. The absolute bioavailability of cefepime after intramuscular injection averaged 82%.
Drug concentrations in cerebrospinal fluid (CSF) and blood plasma in children with bacterial meningitis
** age of patients: 3.1 months – 12 years, average age: 3 years. The dose of the drug is 50 mg/kg body weight when administered intravenously over 5 to 20 minutes every 8 hours. Concentrations in plasma and CSF were determined at the end of administration on days 2 or 3 of drug treatment.
Special instructions
Special instructions
In the presence of factors that can cause renal impairment, cefepime dosage adjustment is required to compensate for the reduced rate of drug excretion in urine. The dosage regimen depends on the degree of renal failure, the severity of the infection and the sensitivity of microorganisms. For mild or moderate renal dysfunction, the initial dose of the drug is the same as for normal renal function. The risk of developing toxic reactions is especially increased in elderly patients with impaired renal function.
During post-marketing surveillance, the following serious adverse reactions, including life-threatening or fatal, were reported: encephalopathy (impaired consciousness, including confusion, hallucinations, stupor and coma), myoclonus, seizures and non-convulsive status epilepticus.
Most cases were observed in patients with renal failure who did not undergo dose adjustment. However, in some cases, neurotoxicity has been observed in patients who received dose adjustments based on the degree of renal impairment. In most cases, symptoms of neurotoxicity were reversible and disappeared after discontinuation of the drug and/or after hemodialysis. If neurotoxicity is associated with cefepime use, consider discontinuing cefepime therapy or adjust the dose in patients with renal impairment. Before starting treatment, it is necessary to establish whether the patient has a history of allergic reactions to cefepime, other cephalosporin antibiotics, penicillins and other beta-lactam antibiotics, as well as other forms of allergies. When using all types of beta-lactam antibiotics, cases of severe hypersensitivity reactions, sometimes fatal, have been reported.
Antibiotics of the cephalosporin group may cause a false-positive reaction to glucose in urine in tests based on the reduction of copper ions (with Benedict’s or Fehling’s solutions or with Clinitest tablets), but not in enzyme tests (with glucose oxidase). In this regard, it is recommended to use enzyme tests with glucose oxidase to determine glucose in urine.
If an allergic reaction develops, treatment with the drug should be stopped and appropriate measures taken. If a severe allergic reaction (eg, anaphylactic reaction) develops immediately during drug administration, the use of epinephrine and other supportive therapy may be required.
When prescribing empirical treatment, it is necessary to take into account data on the acquired resistance of pathogenic microorganisms.
The resistance of microorganisms may change over time and geographical location. To identify the causative microorganism and determine sensitivity to cefepime, appropriate tests should be performed. Cefepime can be used as monotherapy even before identification of the causative microorganism, since it has a wide spectrum of antibacterial action against gram-positive and gram-negative microorganisms. If there is a risk of a mixed aerobic/anaerobic infection (especially when microorganisms insensitive to cefepime may be present), treatment with cefepime in combination with a drug that acts on anaerobes can be started before identification of the pathogen.
After identifying the pathogen and determining antibiotic sensitivity, treatment should be carried out in accordance with the test results.
As with the use of other antibiotics, treatment with Cefepime may lead to the colonization of insensitive microflora. If superinfections develop during treatment, appropriate measures must be taken.
No effects on fertility were noted in rat studies. There are no data on the effects on fertility in humans with cefepime.
Clostridium difficile – associated diarrhea
When using almost all broad-spectrum antibiotics, Clostridium difficile-associated diarrhea (CDAD – Clostridium difficile-associated diarrhea) may occur, which can be either mild or severe, even fatal.
If diarrhea occurs during treatment with the drug, the diagnosis of CDAD must be confirmed. The patient should be closely monitored for the development of CDAD, as cases have been reported of its occurrence more than two months after stopping antibiotic use. If CDAD is suspected or confirmed, use of antibiotics other than those prescribed to suppress Clostridium difficile should be discontinued. You should not use medications that inhibit intestinal motility.
Impact on the ability to drive vehicles and machinery
The effect of the drug on the ability to concentrate has not been studied, however, given the possibility of developing side effects from the central nervous system, during treatment with the drug you should refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Cefepime
Composition
Composition
For 1 bottle:
Active ingredient:
Cefepime hydrochloride monohydrate – 1.1891 g, (in terms of cefepime) – 1.0 g,
Excipient:
L-arginine – 0.725 g.
Pregnancy
Pregnancy
Adequate and controlled clinical studies have not been conducted in pregnant women. During pregnancy, the drug should be used only if the expected benefit to the mother outweighs the potential risk to the fetus.
Cefepime is found in breast milk in very low concentrations.
During breastfeeding, the drug should be used only if the expected benefit to the mother outweighs the potential risk to the child.
Contraindications
Contraindications
Hypersensitivity to cefepime or L-arginine, as well as to cephalosporin antibiotics, penicillins or other beta-lactam antibiotics.
Children up to 2 months of age.
With caution
History of gastrointestinal tract diseases (especially colitis), renal failure (creatinine clearance less than 60 ml/min).
Side Effects
Side Effects
The most commonly reported side effects are gastrointestinal and allergic reactions. Side effects are listed below by organs and systems according to their frequency: very often (≥10%); often (≥1% and <10%); uncommon (≥0.1% and <1%); rare (≥0.01% and <0.1%); frequency unknown (no data on the frequency of development of this side effect).
Allergic reactions
Often – skin rashes;
Uncommon – erythema, urticaria, itching;
Rarely – anaphylactic reactions;
Frequency unknown – anaphylactic shock, toxic epidermal necrolysis, erythema multiforme, Stevens-Johnson syndrome, angioedema.
Infections
Uncommon – candidiasis of the oral mucosa, vaginal infections;
Rarely – candidiasis.
From the central nervous system
Uncommon: headache;
Rarely – convulsions, paresthesia, dysgesia, dizziness;
Frequency unknown (post-marketing experience): encephalopathy (impaired consciousness, including confusion, hallucinations, stupor and coma), myoclonus, convulsions and non-convulsive status epilepticus.
Although most cases were observed in patients with renal impairment who received cefepime at doses higher than recommended, some cases of neurotoxicity were observed in patients who received dose adjustments based on the degree of renal impairment.
From the urinary system
Frequency unknown – renal failure, toxic nephropathy.
From the digestive system
Often – diarrhea;
Uncommon: nausea, vomiting, colitis (including pseudomembranous colitis);
Rarely – abdominal pain, constipation;
Frequency unknown – digestive disorders.
From the respiratory system
Rarely – shortness of breath.
From the cardiovascular system
Rarely – vasodilation;
Frequency unknown – bleeding.
General reactions and reactions at the injection site
Often – phlebitis at the injection site, pain at the injection site;
Uncommon: fever and inflammation at the injection site;
Rarely – chills.
Laboratory indicators
Often – increased activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, anemia, eosinophilia, increased prothrombin time or partial thromboplastin time;
Uncommon: increased blood urea nitrogen, serum creatinine, thrombocytopenia, leukopenia and neutropenia;
Frequency unknown – aplastic anemia, hemolytic anemia, agranulocytosis.
Other
Rarely – genital itching, change in taste, vaginitis, erythema, false-positive Coombs test without hemolysis.
Interaction
Interaction
The drug solution is pharmaceutically incompatible with solutions of metronidazole, vancomycin, gentamicin, tobramycin sulfate, netilmicin sulfate, so they should not be mixed. However, when cefepime and the above drugs are prescribed simultaneously, each of them can be administered separately.
Data on the compatibility of solutions of cefepime and other drugs, as well as their stability, are given in the table below.
Concomitant use with bacteriostatic antibiotics may reduce the effectiveness of beta-lactam antibiotics.
Overdose
Overdose
Symptoms: encephalopathy (confusion, hallucinations, stupor, coma), myoclonic convulsions, increased neuromuscular excitability.
Treatment: symptomatic and supportive therapy. In case of significant excess of recommended doses, especially in patients with impaired renal function, hemodialysis is indicated.
Storage conditions
Storage conditions
In a place protected from light at a temperature not exceeding 30°C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
Deco Company, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 30 ° C. Keep out of reach of children. |
| Manufacturer | Deco Company, Russia |
| Medication form | Powder for preparation of solution |
| Brand | Deco Company |
Other forms…
Related products
Buy Cefepim, 1 g with delivery to USA, UK, Europe and over 120 other countries.