No products in the cart.
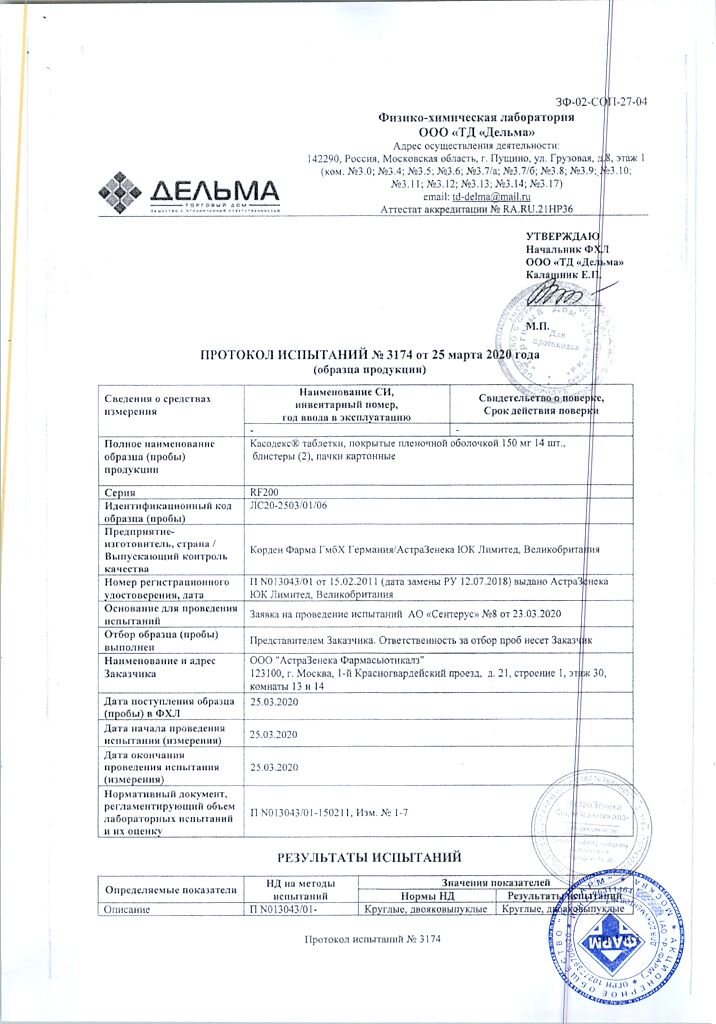
Casodex, 150 mg 28 pcs.
€151.27 €139.63
Description
Pharmacodynamics
An antiandrogenic nonsteroidal drug. It is a racemic mixture, with predominantly the (R)-enantiomer having antiandrogenic activity. The drug has no other types of endocrine activity. Casodex® binds to androgen receptors and, without activating gene expression, suppresses the stimulating effect of androgens. This results in regression of prostate neoplasms.
In some patients, discontinuation of Cassodex may lead to the development of clinical antiandrogen withdrawal syndrome.
When Casodex is used at a daily dose of 150 mg daily to treat patients with locally advanced (T3-T4, any N, M0 or any T, N+, M0) prostate cancer, as immediate hormone therapy or as adjuvant therapy, the risk of disease progression and bone metastases is significantly reduced.
In locally advanced prostate cancer there was a trend toward improved survival with no evidence of disease progression in the patient groups who received Casodex® 150 mg as immediate or adjuvant therapy compared to standard therapy (surgery, radiation therapy).
Life expectancy has been shown to improve among patients with locally advanced prostate cancer who received Casodex® 150 mg as immediate monotherapy and adjuvant therapy in combination with radiotherapy.
The use of Cassodex® at a dose of 150 mg compared to surgical castration in patients with locally advanced nonmetastatic prostate cancer showed no statistically significant difference in survival and time to progression with a statistically significant advantage in sexual function and physical status.
Pharmacokinetics
Intake
After oral administration, it is rapidly and completely absorbed from the gastrointestinal tract. Concomitant ingestion does not affect absorption.
Distribution
Daily administration of Cassodex at a dose of 150 mg Css (R)-enantiomer in plasma is about 22 µg/mL. At equilibrium, about 99% of all circulating enantiomers in the blood are the active (R)-enantiomer.
The plasma concentration of the (R)-enantiomer is increased about 10-fold with daily administration of Cassodex due to the long T1/2, which allows Cassodex to be taken once daily.
The binding to plasma proteins is high (for the racemic mixture 96%, for the (R)-enantiomer 99.6%).
The average concentration of the (R)-enantiomer in the semen of men treated with Casodex 150 mg is 4.9 µg/ml. The amount of bicalutamide potentially found in women after sexual intercourse is low, at approximately 0.3 µg/kg (a value lower than that required for fetal developmental changes in laboratory animals).
Metabolism
It is intensively metabolized in the liver by oxidation and formation of conjugates with glucuronic acid.
Excretion
The metabolites are excreted with urine and bile in approximately equal proportions. The (S)-enantiomer is eliminated much faster than the (R)-enantiomer, the T1/2 of the latter is about 7 days.
Pharmacokinetics in special clinical cases
The pharmacokinetics of (R)-enantiomer is not affected by age, renal impairment and mild to moderate hepatic impairment.
There is evidence that in patients with severe hepatic impairment the elimination of (R)-enantiomer from plasma is delayed.
In patients with moderate to severe hepatic impairment there may be cumulation of bicalutamide in the body.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
1 tablet contains:
The active ingredient:
bicalutamide 150 mg.
Associates:
lactose monohydrate – 183 mg,
sodium carboxymethyl starch – 22.5 mg,
povidone – 15 mg,
magnesium stearate – 4.5 mg.
Composition of the shell:
hypromellose – 7.5 mg,
macrogol 300 – 1.5 mg,
titanium dioxide (E171) – 2.3 mg.
How to take, the dosage
How to take, the dosage
Casodex® is administered to adults and elderly men at a dose of 150 mg once daily. Lasodex® should be taken long-term, for at least 2 years. If there are signs of disease progression, the drug should be stopped.
Patients with impaired renal function and mild hepatic impairment do not require dose adjustment.
In patients with moderate to severe hepatic impairment there may be increased cumulation of the drug.
Interaction
Interaction
There are no data on pharmacodynamic or pharmacokinetic interactions between Casodex and GnRH analogues.
In in vitro studies, the (R)-enantiomer of Cassodex has been shown to be an inhibitor of CYP3A4 and to a lesser extent of CYP2C9, CYP2C19, CYP2D6. No potential ability of Kasodex to interact with other drugs has been found. However, when using Cassodex for 28 days against midazolam, the AUC of midazolam was increased by 80%.
Casodex is contraindicated with such drugs as terfenadine, astemizole and cisapride.
Caution should be exercised when prescribing Casodex together with cyclosporine or calcium channel blockers. It may be necessary to reduce the dose of these drugs, especially in case of potentiation or development of adverse reactions. Careful monitoring of cyclosporine plasma concentrations and the patient’s clinical condition is recommended after initiation or withdrawal of Cassodex.
The concomitant administration of Cassodex and drugs that inhibit microsomal oxidation of drugs (cimetidine, ketoconazole) may lead to increased plasma concentrations of bicalutamide and possibly to an increased incidence of adverse reactions.
Casodex® potentiates the effect of coumarin-type indirect anticoagulants (warfarin) because it can displace these drugs from their binding sites with proteins.
Special Instructions
Special Instructions
Given the possibility of delayed excretion of bicalutamide and cumulation of bicalutamide in patients with impaired liver function, periodic evaluation of liver function is advisable. Most changes in liver function occur during the first 6 months of treatment with Casodex.
If significant changes in liver function develop, Casodex should be discontinued.
In patients with disease progression with elevated prostate-specific antigen (PSA) levels, discontinuation of treatment with Casodex should be considered.
In patients receiving coumarin anticoagulants, regular monitoring of prothrombin time is recommended when prescribing Cassodex.
With regard to possible inhibition of cytochrome P450 (CYP3A4) activity by Xadex, caution should be exercised when co-administering Xadex with drugs that are primarily metabolized with CYP3A4.
Patients with lactose intolerance should be informed that each tablet of Cassodex 150 mg contains 183 mg of lactose monohydrate.
Impact on ability to drive vehicles and other mechanisms requiring high concentration
Drowsiness and dizziness may occur with use of Casodex; therefore, caution should be exercised while driving motor transport or other moving machinery.
Contraindications
Contraindications
With caution: Use Xodex® in patients with hepatic impairment, lactose intolerance, lactase deficiency, and glucose/galactose malabsorption syndrome.
Side effects
Side effects
Except as otherwise noted, the incidence of adverse events is based on studies of monotherapy with Casodex® for early prostate cancer.
Very common (â¥10%): Gynecomastia (may persist even after discontinuation of therapy, especially if the drug has been taken for a long time), breast tenderness, skin rash, asthenia.
Frequently (â¥1%- < 10%): Depression, anorexia, dizziness, somnolence, hot flashes, itching, abdominal pain, constipation, dyspepsia, flatulence, alopecia or hair regrowth, hirsutism, dry skin, hematuria, nausea, reduced libido, erectile dysfunction, breast pain, edema, weight gain, increased liver transaminase activity, hepatotoxicity, jaundice, anemia, decreased appetite.
Infrequent (â¥0.1%- < 1%): hypersensitivity reactions, angioedema, urticaria, interstitial lung disease (fatal cases reported)*.
Rarely (â¥0.01%- < 0.1%): hepatic failure (fatal cases have been reported)*
Elevated liver transaminases, cholestasis and jaundice were rarely rated as serious, were transient, completely disappeared or decreased with continued therapy or after drug withdrawal. Very rarely, liver failure developed during bicalutamide therapy, but a causal relationship between the development of liver failure and treatment with Casodex has not been reliably established.
* – Based on post-marketing use of the drug.
Overdose
Overdose
No cases of overdose in humans have been described.
There is no specific antidote, so if necessary symptomatic therapy is carried out.
Dialysis is ineffective because bicalutamide is firmly bound to proteins and is not excreted unchanged in the urine.
General supportive therapy and monitoring of vital body functions are indicated.
Pregnancy use
Pregnancy use
Casodex® is contraindicated in women and should not be administered to pregnant or lactating women.
Similarities
Similarities
Additional information
| Weight | 0.022 kg |
|---|---|
| Shelf life | 4 years |
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Corden Pharma GmbH, Germany |
| Medication form | pills |
| Brand | Corden Pharma GmbH |
Other forms…
Related products
Buy Casodex, 150 mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.