Subtotal: €60.51
Carsil Forte, 90 mg capsules 30 pcs
€11.43 €9.53
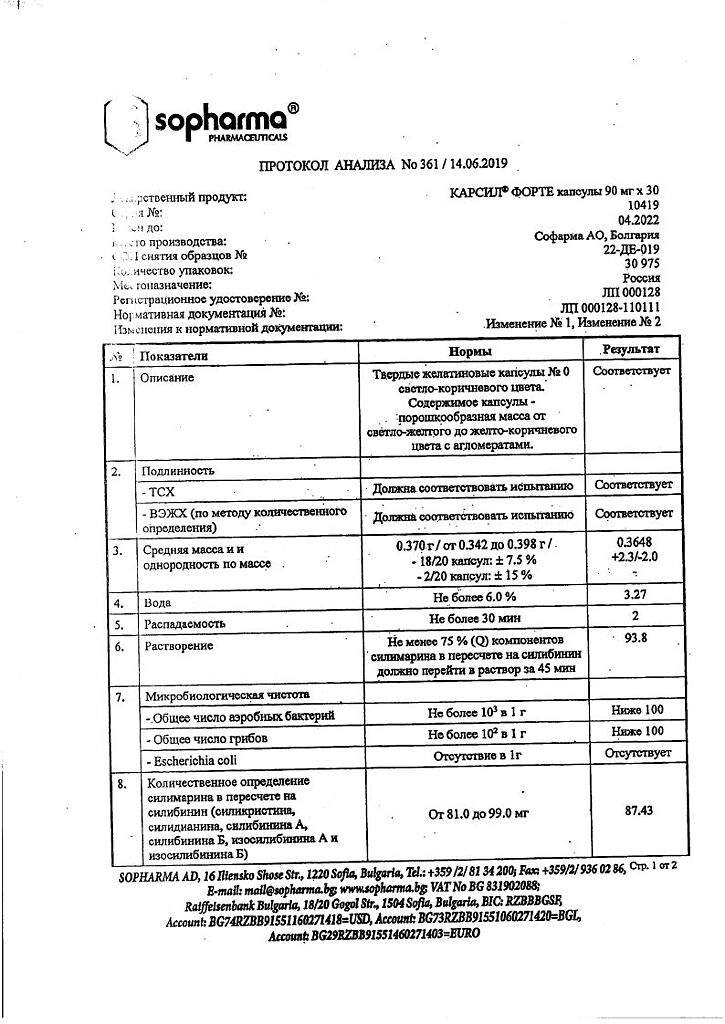
Carsil Forte belongs to the group of hepatoprotective drugs. It contains a dry extract of milk thistle fruits (equivalent of silymarin), which is a mixture of 4 isomers of flavonolignans: silybinin, isosilybinin, silydianin and silycristin.
The mechanism of action of the drug is still not well elucidated. It is established that the hepatoprotective effect of silymarin is conditioned by competitive interaction with toxins for corresponding receptors in membrane of hepatocytes, i. e. by membrane stabilizing action.
Silymarin has metabolic and cell-regulating effects, influencing cell membrane permeability by inhibiting the 5-lipoxygenase pathway, especially LT B4, and by binding to free reactive oxygen radicals. Stimulates the synthesis of proteins (structural and functional) and phospholipids in the affected hepatocytes, accelerating regenerative processes. The action of flavonoids, to which belongs silymarin, is also due to their antioxidant and improving microcirculation effects. Clinically, these effects are expressed in the improvement of subjective and objective symptoms and normalization of liver function parameters (transaminases, γ-globulin, bilirubin). This leads to improvement of general state, reduction of digestive complaints, and in patients with poor digestion due to liver disease leads to increase of appetite and body weight gain.
Pharmacokinetics
Absorption is low and slow. Subject to intestinal-hepatic recirculation. Does not cumulate.
In studies of C14-labeled silibinin, the highest concentrations are found in the liver and very small amounts in the kidneys, lungs, heart and other organs.
It is metabolized in the liver by conjugation. Glucuronides and sulfates are found as metabolites in bile.
T1/2 is 6 hours. It is excreted mainly with the bile (about 80%) as glucuronides and sulfates and to a small extent (about 5%) with the urine.
Indications
As part of complex therapy for the following conditions and diseases:
toxic liver damage;
conditions after acute hepatitis;
chronic hepatitis of non-viral etiology;
liver steatosis (non-alcoholic and alcoholic);
cirrhosis;
prevention of liver damage due to prolonged use of medications, alcohol, chronic intoxication (including occupational).
Pharmacological effect
Karsil Forte belongs to the group of hepatoprotective drugs. Contains milk thistle fruit dry extract (silymarin equivalent), which is a mixture of 4 flavonolignan isomers: silibinin, isosilibinin, silydianin and silicristin.
The mechanism of action of the drug is still not well understood. It has been established that the hepatoprotective effect of silymarin is determined by competitive interaction with toxins for the corresponding receptors in the hepatocyte membrane, i.e. membrane-stabilizing effect.
Silymarin has metabolic and cell-regulating effects, affects the permeability of the cell membrane, suppressing the 5-lipoxygenase pathway, especially LT B4, and also by binding to free reactive oxygen radicals. Stimulates the synthesis of proteins (structural and functional) and phospholipids in affected hepatocytes, accelerating regenerative processes. The action of flavonoids, to which silymarin belongs, is also determined by their antioxidant and microcirculation-improving effects. Clinically, these effects are expressed in the improvement of subjective and objective symptoms and normalization of indicators of the functional state of the liver (transaminases, γ-globulin, bilirubin). This leads to an improvement in general condition, a reduction in complaints related to digestion, and in patients with reduced absorption of food due to liver disease, it leads to an increase in appetite and weight gain.
Pharmacokinetics
Absorption is low and slow. Subject to enterohepatic recirculation. Does not cumulate.
When studying C14-labeled silibinin, the highest concentrations are found in the liver and very small amounts in the kidneys, lungs, heart and other organs.
Metabolized in the liver through conjugation. Glucuronides and sulfates are found in bile as metabolites.
T1/2 is 6 hours. It is excreted mainly in bile (about 80%) in the form of glucuronides and sulfates and to a small extent (about 5%) in urine.
Special instructions
Impact on the ability to drive vehicles and operate machinery.
The use of the drug in monotherapy does not affect the ability to drive vehicles or operate machinery.
Active ingredient
Milk thistle fruit extract
Composition
1 capsule contains:
active ingredient:
milk thistle fruit dry extract 163.6–225 mg (equivalent to silymarin content 90 mg)
excipients:
lactose monohydrate – 38.2–7.5 mg;
MCC (type 101) – 38.2–7.5 mg;
wheat starch – 15.5 mg;
povidone K25 – 3.7 mg;
polysorbate 80 – 3.7 mg;
colloidal silicon dioxide anhydrous – 3.4 mg;
mannitol – 80 mg;
crospovidone – 14 mg;
sodium bicarbonate – 6 mg;
magnesium stearate – 3.7 mg,
capsule:
iron oxide black – 0.02%;
iron oxide red – 0.03%;
titanium dioxide – 0.6666%;
iron oxide yellow – 0.35%;
gelatin – up to 100%.
Pregnancy
It is not recommended to use the drug during pregnancy and breastfeeding.
Contraindications
hypersensitivity to the active or any of the excipients of Carsil;
lactase deficiency, galactosemia or glucose/galactose malabsorption syndrome (due to the presence of lactose in the drug);
celiac disease (gluten enteropathy) – due to the presence of wheat starch in the composition of the drug;
children under 12 years of age.
With caution: patients with hormonal disorders (endometriosis, uterine fibroids, breast, ovarian and uterine carcinoma, prostate carcinoma) – an estrogen-like effect of silymarin may occur.
Side Effects
The drug is well tolerated. The following side effects are rarely observed.
From the gastrointestinal tract: nausea, dyspepsia, diarrhea.
On the skin: in isolated cases, allergic skin reactions are possible – itching, rash, alopecia.
Others: It is rare to see an exacerbation of existing vestibular disorders.
Side effects are transient and disappear after stopping the drug.
Interaction
When silymarin is used together with oral contraceptives and drugs used in hormone replacement therapy, the effects of the latter may be reduced.
Silymarin may enhance the effects of drugs such as diazepam, alprazolam, ketoconazole, lovastatin, vinblastine due to its inhibitory effect on the cytochrome P450 system.
Overdose
There is no evidence of drug overdose.
Treatment for accidental ingestion of a high dose: induction of vomiting, gastric lavage, use of activated charcoal, and symptomatic therapy if necessary.
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
2 years
Manufacturer
Sopharma JSC, Bulgaria
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Sofarma JSC, Bulgaria |
| Medication form | capsules |
| Brand | Sofarma JSC |
Related products
Buy Carsil Forte, 90 mg capsules 30 pcs with delivery to USA, UK, Europe and over 120 other countries.