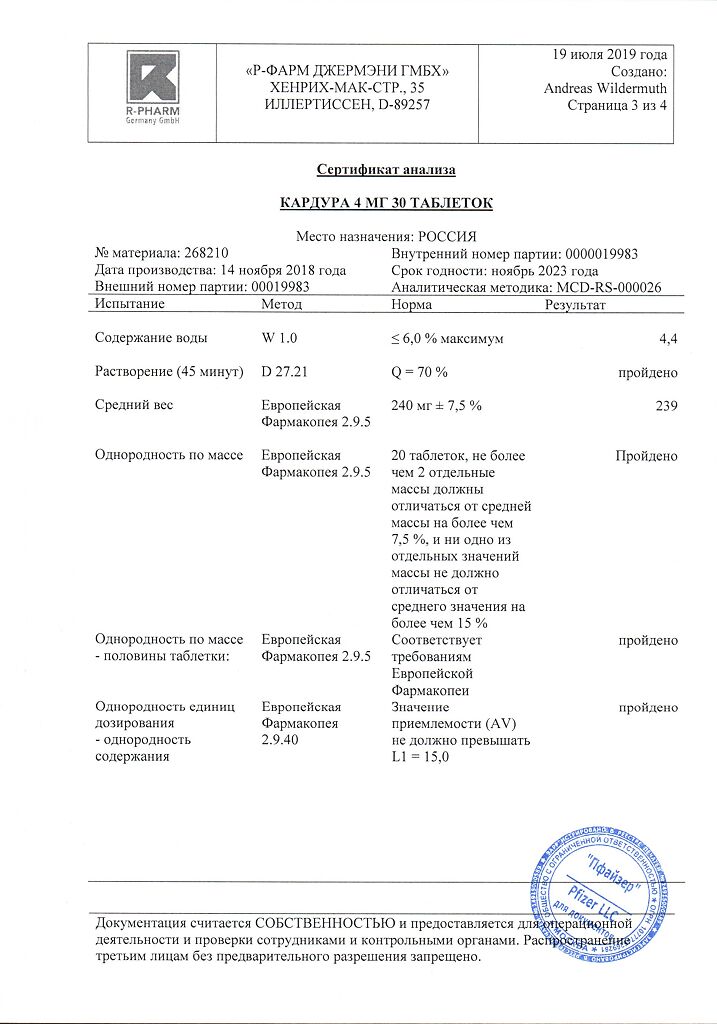
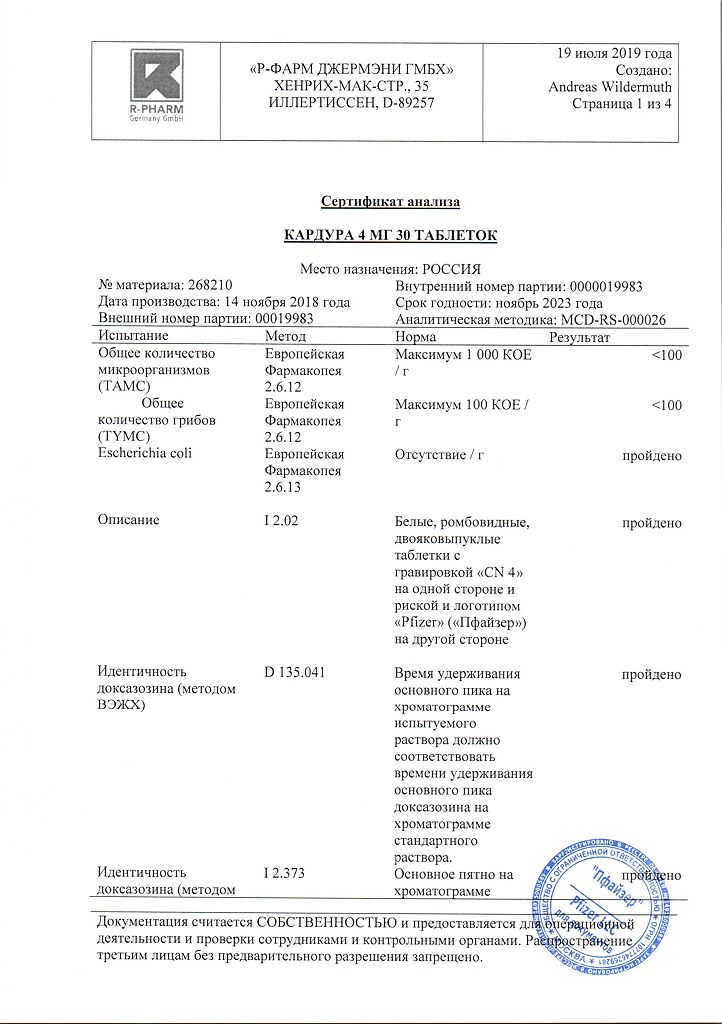
No products in the cart.
Cardura, tablets 4 mg 30 pcs
€8.81 €7.71
Out of stock
(E-mail when Stock is available)
Description
Cardura is a vasodilator.
Pharmacodynamics
Participation of doxazosin in patients with symptoms of BPH leads to significant improvement of urodynamic parameters and reduction of symptoms of the disease. This action of the drug is explained by selective blockade of alpha1-adrenoreceptors located in stroma and capsule of prostate, bladder neck.
Doxazosin has been shown to be an effective blocker of subtype 1A alpha1 adrenoreceptors, which represent approximately 70% of all subtypes, alpha1 adrenoreceptors located in the prostate. This explains its action in patients with BPH.
The maintenance effect of treatment with doxazosin and its safety have been proven with long-term use of the drug (e.g., up to 48 months).
Arterial hypertension
The use of doxazosin in patients with arterial hypertension leads to a significant decrease in BP as a result of reduction of PPS. Appearance of this effect is associated with selective blockade of alpha1-adrenoreceptors located in vascular network. When administered once daily, the clinically significant antihypertensive effect is maintained for 24 hours, BP decreases gradually; the maximum effect is usually observed 2-6 hours after oral administration. In patients with arterial hypertension BP during doxazosin treatment was similar in the supine and standing positions.
It was noted that in contrast to non-selective alpha1-adreno-blockers, tolerance to the drug did not develop during long-term treatment with doxazosin. Increased plasma renin activity and tachycardia are infrequent during maintenance therapy.
Doxazosin has favorable effect on blood lipid profile, significantly increasing HDL to total cholesterol ratio and significantly decreasing total triglycerides and total cholesterol. In this regard, it has an advantage over diuretics and beta-adrenoblockers, which have no favorable effect on these parameters. Taking into account the established connection of arterial hypertension and blood lipid profile with CHD, normalization of BP and lipid concentration during doxazosin administration leads to decrease of CHD risk.
It was observed that doxazosin treatment resulted in regression of left ventricular hypertrophy, inhibition of platelet aggregation and enhancement of tissue plasminogen activator activity. In addition, doxazosin was found to increase insulin sensitivity in patients with impaired glucose tolerance.
Doxazosin has no metabolic side effects and can be used in patients with bronchial asthma, diabetes, left ventricular insufficiency and gout.
In vitro studies have demonstrated the antioxidant properties of 6′- and 7′- hydroxymetabolites of doxazosin at a concentration of 5 μmol.
In controlled clinical studies conducted in patients with arterial hypertension, treatment with doxazosin was accompanied by improvement of erectile function. In addition, patients treated with doxazosin had less recurrence of erectile dysfunction than patients treated with antihypertensives.
Pharmacokinetics
Doxazosin is well absorbed after oral administration at therapeutic doses; Tmax in blood is reached after approximately 2 h.
Doxazosin is approximately 98% bound to plasma proteins.
The primary pathways of metabolism of doxazosin are O-demethylation and hydroxylation.
The plasma excretion is biphasic, with a final T1/2 of 22 h, allowing the drug to be administered once daily. Doxazosin undergoes active biotransformation; only less than 5% of the dose is excreted unchanged.
Performance in Special Patient Groups
According to pharmacokinetic studies, the pharmacokinetics of the drug in elderly patients and patients with renal impairment do not differ significantly from those in younger patients with normal renal function.
There are limited data from patients with impaired hepatic function about the effects of drugs that can alter hepatic metabolism (e.g., cimetidine). In a clinical study in 12 patients with moderate hepatic impairment, a single use of doxazosin was accompanied by a 43% increase in AUC and a 40% decrease in true oral clearance. Caution should be exercised when prescribing doxazosin, as well as other drugs fully biotransformed in the liver, in patients with liver dysfunction (see “Cautions”).
Indications
Indications
– benign prostatic hyperplasia;
– for the treatment of urinary retention and symptoms associated with BPH;
– arterial hypertension (as part of combination therapy).
Pharmacological effect
Pharmacological effect
Benign prostatic hyperplasia
Prescribing doxazosin to patients with symptoms of benign prostatic hyperplasia (BPH) leads to a significant improvement in urodynamics and a decrease in the manifestations of symptoms of the disease. This effect of the drug is associated with the selective blockade of alpha1-adrenergic receptors located in the stroma and capsule of the prostate gland, and the neck of the bladder.
Doxazosin has been shown to be an effective blocker of subtype 1A alpha1-adrenergic receptors, which constitute approximately 70% of all alpha1-adrenergic receptor subtypes found in the prostate gland. This explains its effect in patients with BPH.
The supporting effect of doxazosin treatment and its safety have been proven with long-term use of the drug (for example, up to 48 months).
Arterial hypertension
The use of doxazosin in patients with arterial hypertension leads to a significant decrease in blood pressure (BP) as a result of a decrease in total peripheral vascular resistance. The appearance of this effect is associated with selective blockade of alpha1-adrenergic receptors located in the vascular network. When taking the drug once a day, a clinically significant antihypertensive effect persists for 24 hours, blood pressure decreases gradually; the maximum effect is usually observed 2-6 hours after taking the drug orally. In patients with arterial hypertension, blood pressure during treatment with doxazosin was the same in the “lying” and “standing” positions.
It was noted that, in contrast to non-selective alpha1-blockers, tolerance to the drug did not develop during long-term treatment with doxazosin. During maintenance therapy, increased plasma renin activity and tachycardia are uncommon.
Doxazosin has a beneficial effect on the blood lipid profile, significantly increasing the ratio of high-density lipoprotein to total cholesterol and significantly reducing total triglycerides and total cholesterol. In this regard, it has an advantage over diuretics and beta-blockers, which do not have a beneficial effect on these parameters. Considering the established connection between arterial hypertension and blood lipid profile and coronary heart disease, normalization of blood pressure and lipid concentrations while taking doxazosin leads to a reduction in the risk of developing coronary heart disease.
It was observed that treatment with doxazosin resulted in regression of left ventricular hypertrophy, inhibition of platelet aggregation, and increased tissue plasminogen activator activity. In addition, doxazosin has been found to improve insulin sensitivity in patients with impaired glucose tolerance.
Doxazosin has no metabolic side effects and can be used in patients with bronchial asthma, diabetes mellitus, left ventricular failure and gout.
In vitro studies have shown the antioxidant properties of 6′ and 7′ – hydroxymetabolites of doxazosin at a concentration of 5 micromoles.
In controlled clinical studies conducted in patients with arterial hypertension, treatment with doxazosin was accompanied by an improvement in erectile function.
In addition, in patients receiving doxazosin, new erectile dysfunction was observed less frequently than in patients receiving antihypertensive drugs.
After oral administration in therapeutic doses, doxazosin is well absorbed; its concentration in the blood reaches a maximum after about 2 hours. Doxazosin is approximately 98% bound to plasma proteins.
The primary metabolic pathways of doxazosin are O-demethylation and hydroxylation.
Elimination from blood plasma is biphasic with a final half-life of 22 hours, which allows the drug to be administered once a day. Doxazosin undergoes active biotransformation in the liver. In vitro studies have shown that the main route of elimination of doxazosin is through the CYP3A4 isoenzyme; however, the elimination pathways through the CYP2D6 and CYP2C9 isoenzymes are also involved in the process, but to a lesser extent. Only less than 5% of the dose is excreted unchanged.
Use in special patient groups
According to pharmacokinetic studies in elderly patients and patients with renal failure, the pharmacokinetics of the drug do not differ significantly from that in younger patients with normal renal function.
There are only limited data obtained in patients with impaired liver function and on the effect of drugs that can alter hepatic metabolism (for example, cimetidine). In a clinical study in 12 patients with moderate hepatic impairment, a single dose of doxazosin was associated with an increase in the area under the concentration-time curve (AUC) by 43% and a decrease in true oral clearance by 40%. Caution must be exercised when prescribing doxazosin, as well as other drugs that are completely bitransformed in the liver, to patients with impaired liver function (see section “Special Instructions”).
Special instructions
Special instructions
Postural hypotension/syncope
As with treatment with any alpha-blockers, especially at the beginning of therapy, a small percentage of patients experienced postural hypotension, manifested by dizziness and weakness or loss of consciousness (fainting) (see section “Dosage and Administration”). Before prescribing any alpha-blocker, the patient must be warned how to avoid symptoms of postural hypotension, in particular, it is necessary to refrain from rapid changes in body position. At the beginning of treatment with Cardura, the patient should be advised to exercise caution if weakness or dizziness occurs.
Cardura should be used with caution in elderly patients due to the possibility of developing orthostatic hypotension. As you age, your risk of dizziness, blurred vision and fainting increases.
The patient should be informed that the risk of developing orthostatic hypotension increases with alcohol consumption, prolonged standing or exercise, and hot weather.
Benign prostatic hyperplasia
In patients with BPH, the drug can be prescribed both in the presence of arterial hypertension and normal blood pressure. When using the drug in patients with BPH with normal blood pressure, the change in the latter is insignificant. At the same time, in patients with a combination of arterial hypertension and BPH, use in monotherapy is possible.
Before starting treatment for prostate hyperplasia, it is necessary to exclude its cancerous degeneration.
Doxazosin does not affect the concentration of prostate-specific antigen (PSA) in blood plasma.
Intraoperative atonic iris syndrome
Intraoperative atonic iris syndrome (a variant of the “narrow pupil” syndrome) has been observed in some patients undergoing cataract surgery who are receiving or have received treatment with alpha1-blockers. Since intraoperative atonic iris syndrome can lead to increased complications during surgical interventions, it is necessary to warn the surgeon that alpha1-blockers are currently being taken or have been taken previously before surgery.
Combined use with PDE-5 inhibitors
Caution should be exercised when co-administering Cardura with PDE5 inhibitors as this may result in symptomatic hypotension in some patients.
Liver dysfunction
Caution must be exercised when prescribing Cardura, as well as other drugs that are completely biotransformed in the liver, to patients with impaired liver function (see section “Pharmacokinetics”), avoiding prescribing maximum doses.
Priapism
During post-marketing studies, cases of prolonged erection and priapism have been reported during therapy with alpha1-adrenergic receptors, including doxazosin. If an erection persists for more than 4 hours, you should immediately seek medical help. If priapism therapy is not carried out immediately, it can lead to damage to the tissue of the penis and irreversible loss of potency.
Active ingredient
Active ingredient
Doxazosin
Composition
Composition
Active ingredient:
doxazosin 4 mg (as doxazosin mesylate 4.850 mg).
Excipients:
Sodium carboxymethyl starch 2.4 mg, lactose monohydrate 80.0 mg, microcrystalline cellulose* 150.350 mg, magnesium stearate 2.16 mg, sodium lauryl sulfate 0.24 mg.
*The amount may vary slightly depending on the content of the active substance.
Pregnancy
Pregnancy
Although the drug did not have a teratogenic effect in animal experiments, when it was used in extremely high doses, a decrease in fetal survival was observed. These doses were approximately 300 times higher than the maximum recommended doses for humans. There has been a documented case of doxazosin passing into human breast milk. Studies in laboratory animals have shown that doxazosin accumulates in milk. Due to the lack of adequate well-controlled studies in pregnant or lactating women, the safety of Cardura during pregnancy or breastfeeding has not yet been established. In this regard, during pregnancy or lactation, Cardura can be used only when, in the opinion of the doctor, the potential benefit to the mother outweighs the potential risk to the fetus or child.
Contraindications
Contraindications
– hypersensitivity to quinazolines, doxazosin or any of the auxiliary components of the drug;
– lactase deficiency, lactose intolerance, glucose-galactose malabsorption;
– age up to 18 years;
– severe liver failure due to lack of experience in this category of patients;
– urinary tract infections;
– anuria;
– progressive renal failure;
– hypotension and a tendency to orthostatic disorders (including a history);
– concomitant obstruction of the upper urinary tract;
– stones in the bladder.
Side Effects
Side Effects
The frequency of adverse reactions is presented according to the following classification:
Very common: ≥10%
Frequent: ≥1% and <10%
Uncommon: ≥0.1% and <1%
Rare: ≥0.01% and <0.1%
Very rare: <0.01%
Benign prostatic hyperplasia
According to controlled clinical studies, patients with BPH experienced the same adverse reactions as patients with arterial hypertension.
The following adverse reactions have been reported during post-marketing use of the drug:
From the hematopoietic and lymphatic system: very rare – leukopenia, thrombocytopenia.
From the organ of hearing and vestibular apparatus: infrequent – tinnitus.
On the part of the organ of vision: frequent – impaired color perception, infrequent – atonic iris syndrome.
From the gastrointestinal tract: frequent – abdominal pain, diarrhea, dyspepsia, dry oral mucosa; infrequent – flatulence, constipation, vomiting. From the liver: very rare – cholestasis, hepatitis, jaundice.
From the immune system: very rare – anaphylactic reactions. Laboratory indicators: infrequent – weight gain; very rare – increased activity of “liver” transaminases.
From the side of metabolism: infrequent – anorexia.
From the musculoskeletal system: uncommon – arthralgia, back pain, muscle spasms, muscle weakness, myalgia.
From the central and peripheral nervous system: frequent – paresthesia; infrequent – hypoesthesia, tremor.
From the mental side: frequent – agitation, anxiety, insomnia; infrequent – depression.
From the urinary tract: infrequent – increased frequency of urination, polyuria, urinary incontinence; very rare – dysuria, hematuria, nocturia.
From the reproductive system: very rare – gynecomastia, impotence, priapism; very rarely – retrograde ejaculation.
From the respiratory system: frequent – shortness of breath, rhinitis; infrequent – cough, nosebleeds; very rare – exacerbation of existing bronchospasm.
From the skin: uncommon – alopecia, itching, skin rash, purpura; very rare – urticaria.
From the cardiovascular system: infrequent – “flushes” of blood to the skin of the face, marked decrease in blood pressure, postural hypotension. Other: infrequent – pain of various localization.
Arterial hypertension
In controlled clinical trials of Cardura, the most common adverse reactions were classified as postural (occasionally associated with syncope) or non-specific, which included:
From the organ of hearing and vestibular apparatus: frequent – vertigo.
From the gastrointestinal tract: frequent – nausea.
From the central and peripheral nervous system: very common – dizziness, headache; frequent – postural dizziness (after taking the first dose, a pronounced decrease in blood pressure may develop, which can lead to orthostatic dizziness, in severe cases, especially when quickly moving from the “lying” position to the “standing” position or to the “sitting” position – to fainting), drowsiness.
From the respiratory system: frequent – rhinitis.
Other: common – asthenia, swelling of the lower extremities, fatigue, weakness. The following adverse reactions were noted during the marketing use of the drug Cardura in patients with arterial hypertension, although in general such symptoms could be observed in the absence of treatment with this drug: frequent – tachycardia, palpitations, chest pain; uncommon – angina pectoris, myocardial infarction and arrhythmias, very rare – bradycardia, cerebrovascular accidents.
Interaction
Interaction
Concomitant use of Cardura with PDE5 inhibitors may lead to symptomatic hypotension in some patients (see section “Special Instructions”).
In vitro studies have shown that doxazosin is a substrate of the CYP3A4 isoenzyme. Caution should be exercised when doxazosin is used concomitantly with strong CYP3A4 inhibitors such as clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin or voriconazole (see section “Pharmacological properties”).
Most (98%) of doxazosin in blood plasma is protein bound. In vitro studies of human plasma indicate that doxazosin does not affect the protein binding of digoxin, warfarin, phenytoin or indomethacin. In clinical practice, Cardura has been used without any evidence of interaction with thiazide diuretics, furosemide, beta-blockers, antibiotics, oral hypoglycemic agents, uricosurics and anticoagulants.
Nonsteroidal anti-inflammatory drugs (especially indomethacin), estrogens and sympathomimetic agents may reduce the antihypertensive effect of doxazosin. Doxazosin, eliminating the alpha-adrenergic stimulating effects of epinephrine, can lead to the development of tachycardia and arterial hypotension.
When taken concomitantly with sildenafil for the treatment of pulmonary hypertension, the risk of orthostatic hypotension increases.
With a single use of the drug Cardura 1 mg per day for 4 days while taking 400 mg of cimetidine 2 times a day, there was a 10% increase in the average AUC values and a statistically insignificant increase in the average Cmax level (maximum concentration in blood plasma) and the average half-life of doxazosin. This 10% increase in mean AUC values for doxazosin with cimetidine is within the variability (27%) of mean AUC values for doxazosin compared with placebo.
When used simultaneously with other antihypertensive drugs, the severity of their action increases (dose adjustment is necessary).
It is not recommended to take simultaneously with other alpha-adrenergic blockers.
When used simultaneously with inducers of microsomal oxidation in the liver, the effectiveness of doxazosin may increase, and with inhibitors – a decrease.
Overdose
Overdose
Symptoms: pronounced decrease in blood pressure, sometimes accompanied by fainting.
Treatment: it is necessary to immediately place the patient on his back and raise his legs, and, if necessary, carry out symptomatic therapy. The binding of doxazosin to plasma proteins is high, so dialysis is not effective.
Storage conditions
Storage conditions
At a temperature not exceeding 30C.
Keep out of the reach of children.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
USA
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 30 °C |
| Manufacturer | Pfizer, Puerto Rico |
| Medication form | pills |
| Brand | Pfizer |
Other forms…
Related products
Buy Cardura, tablets 4 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.