No products in the cart.
Broncho-Vaxom for children, capsules 3.5mg 30 pcs
€37.11 €32.85
Description
Broncho-Vaxom for children stimulates the immune defense of the body and increases resistance to infections of the respiratory system.
Immunopharmacological studies have shown that Broncho-Vaxom children has the following effects:
- Augments the formation of immunoglobulin A (IgA) secreted by respiratory tract mucosa and saliva.
- Augments the number of circulating T-lymphocytes.
Clinically, Broncho-Vaxom for children reduces the frequency of acute respiratory tract infections, reduces the duration of their course, and reduces the likelihood of exacerbations of chronic bronchitis.
The necessity of other medicines, especially antibiotics is decreased.
Indications
Indications
The drug is used for children aged 6 months to 12 years for:
– prevention of recurrent respiratory tract infections and exacerbations of chronic bronchitis;
– complex treatment of acute respiratory tract infections.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Immunostimulating agent.
ATX code: L03AX
Pharmacological properties
Pharmacodynamics
Immunobiological effect: Broncho-Vaxom® for children causes an immune response in the mucous membrane of the digestive tract. The effect is especially pronounced in Peyer’s patches (PB) of the small intestine. Antigen presenting cells (APCs) in the PB are activated by bacterial lysate and subsequently stimulate cells responsible for specific immunity.
When using the drug Broncho-Vaxom® for children, an increase in the number of circulating B-lymphocytes is observed.
After stimulation of B lymphocytes, there is an increase in the production of polyclonal antibodies, especially serum IgG and IgA secreted by the mucous membrane of the respiratory tract and salivary glands.
These antibodies are the first line of defense against a range of infectious agents (viruses and bacteria).
The drug Broncho-Vaxom® for children has a powerful stimulating effect on the majority of leukocytes, as evidenced by an increase in the number of myeloid and lymphoid cells, as well as a selective increase in the expression of receptors on the surface.
Taken together, these data confirm that the drug Broncho-Vaxom® for children triggers biological reactions that enhance the body’s immune defense against respiratory tract infections.
Clinically, Broncho-Vaxom® for children reduces the frequency of acute respiratory tract infections, shortens the duration of their course, reduces the likelihood of exacerbations of chronic bronchitis, and also increases the body’s resistance to respiratory system infections. This reduces the need to use other medications, especially antibiotics.
Special instructions
Special instructions
To prevent overdose, children should not use the drug from capsules intended for adults (Broncho-Vaxom® adult capsules 7 mg).
Components of the drug may cause a hypersensitivity reaction.
In case of persistent gastrointestinal disorders, skin reactions, respiratory disorders or other symptoms of intolerance to the drug, treatment should be discontinued, because These symptoms are a manifestation of allergic reactions.
Broncho-Vaxom® for children should not be used in children under 6 months of age.
Impact on the ability to drive vehicles and machinery
Broncho-Vaxom® for children does not affect the ability to drive vehicles and machines.
Active ingredient
Active ingredient
Bacterial lysates
Composition
Composition
One capsule contains:
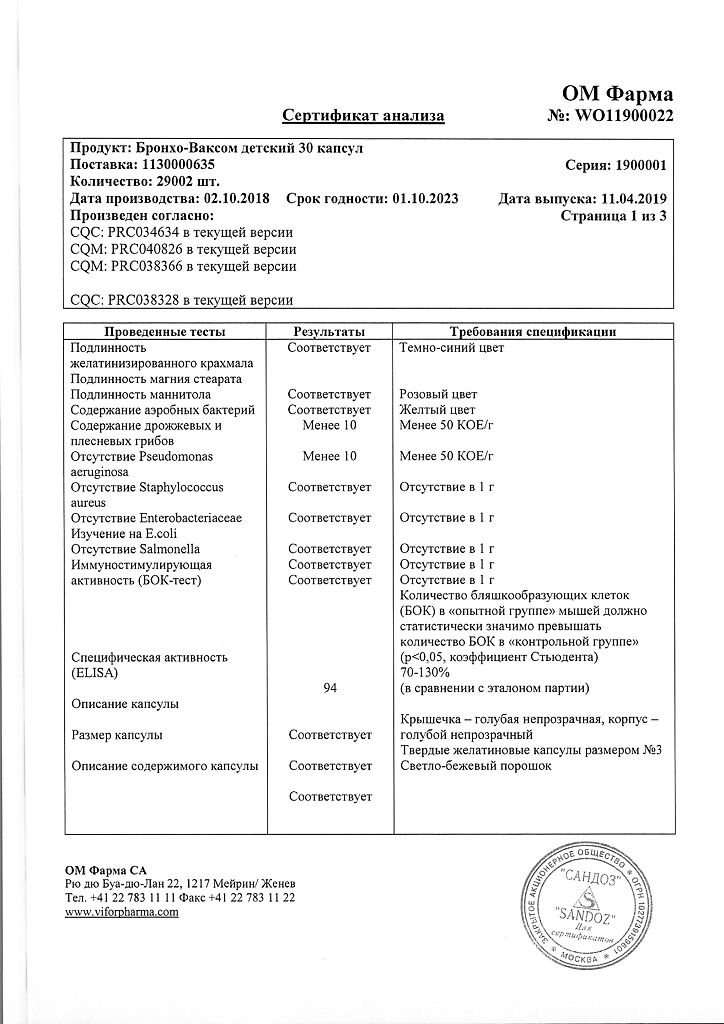
Active ingredient: lyophilisate of bacterial lysates (Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, Klebsiella ozaenae, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus viridans, Moraxella catarrhalis) – 3.50 mg in the form of a standardized OM-85 lyophilisate * – 20.00 mg;
Excipients: pregelatinized starch – 110.00 mg, magnesium stearate – 3.00 mg, mannitol – up to 200.00 mg.
Capsule shell: indigo carmine – 0.01 mg, titanium dioxide – 1.00 mg, gelatin – up to 50.00 mg.
* Standardized OM-85 lyophilisate contains:
Active ingredient: lyophilisate of bacterial lysates (Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, Klebsiella ozaenae, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus viridans, Moraxella catarrhalis) – 3.50 mg.
Excipients: anhydrous propyl gallate – 0.042 mg, anhydrous sodium glutamate – 1.515 mg, mannitol – up to 20.00 mg.
Pregnancy
Pregnancy
The drug is contraindicated in children under 6 months.
Contraindications
Contraindications
Hypersensitivity to the active component or to any of the auxiliary components of the drug.
Side Effects
Side Effects
Broncho-Vaxom® for children is usually well tolerated. Most adverse reactions are classified into the general category with moderate or moderate-severe severity. The most common side effects are gastrointestinal disorders, skin reactions, and respiratory disorders.
In each particular category, side effects are grouped by system-organ class and presented in descending order of frequency:
Very common (≥1/10)
Often (1/100 to 1/10)
Uncommon (1/1,000 to 1/100)
Rarely (from 1/10,000 to 1/1,000)
Very rare (< 1/10000)
Unknown (cannot be estimated based on available data)
Gastrointestinal disorders:
Common: diarrhea, abdominal pain
Unknown: vomiting, nausea
Nervous system disorders:
Unknown: headache.
Disorders of the respiratory system, chest organs, mediastinum:
Common: cough
Disorders of the skin and subcutaneous tissue:
Common: rash
Unknown: urticaria, angioedema
General disorders and disorders at the injection site:
Unknown: fever, fatigue.
Immune system disorders:
Uncommon: hypersensitivity (erythematous rash, generalized rash, erythema, edema, eyelid edema, facial edema, peripheral edema, swelling, facial swelling, pruritus, generalized pruritus, shortness of breath)
Interaction
Interaction
Broncho-Vaxom® for children can be used simultaneously with other medications for the treatment of acute and chronic respiratory diseases.
The interaction of the drug Broncho-Vaxom® for children with other drugs has not yet been established.
Overdose
Overdose
There are no data on cases of overdose. The nature of Broncho-Vaxom® for children and the results of studies of its toxicity in animals indicate that an overdose is unlikely.
Specifications
Specifications
Lyophilisate of bacterial lysates of Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus viridans, Streptococcus pyogenes, Klebsiella pneumoniae, Klebsiella ozaenae, Staphylococcus aureus, Moraxella catarrhalis, used in the drug Broncho-Vaxom® for children, is a bacterial extract containing lyophilized fractions of 21 strains inactivated bacteria belonging to eight different species. The drug Broncho-Vaxom® for children has an immunostimulating effect and enhances the body’s immune defense against respiratory tract infections.
Storage conditions
Storage conditions
At 15–25 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
OM Pharma, Switzerland
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At 15-25 °C |
| Manufacturer | OM Pharma, Switzerland |
| Medication form | capsules |
| Brand | OM Pharma |
Related products
Buy Broncho-Vaxom for children, capsules 3.5mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.