No products in the cart.
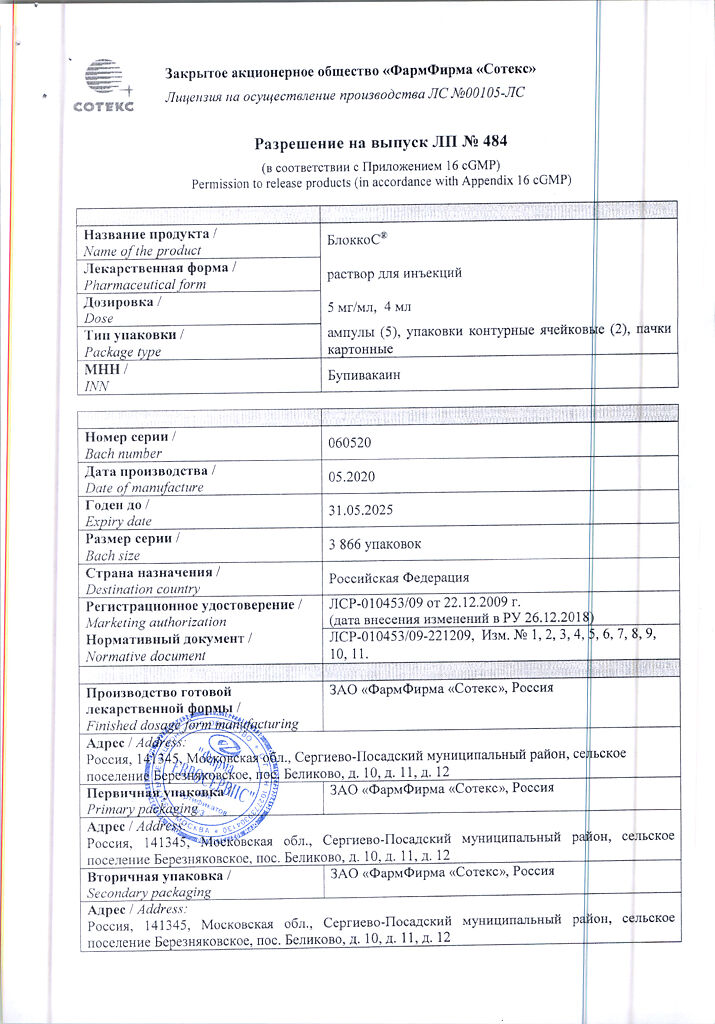
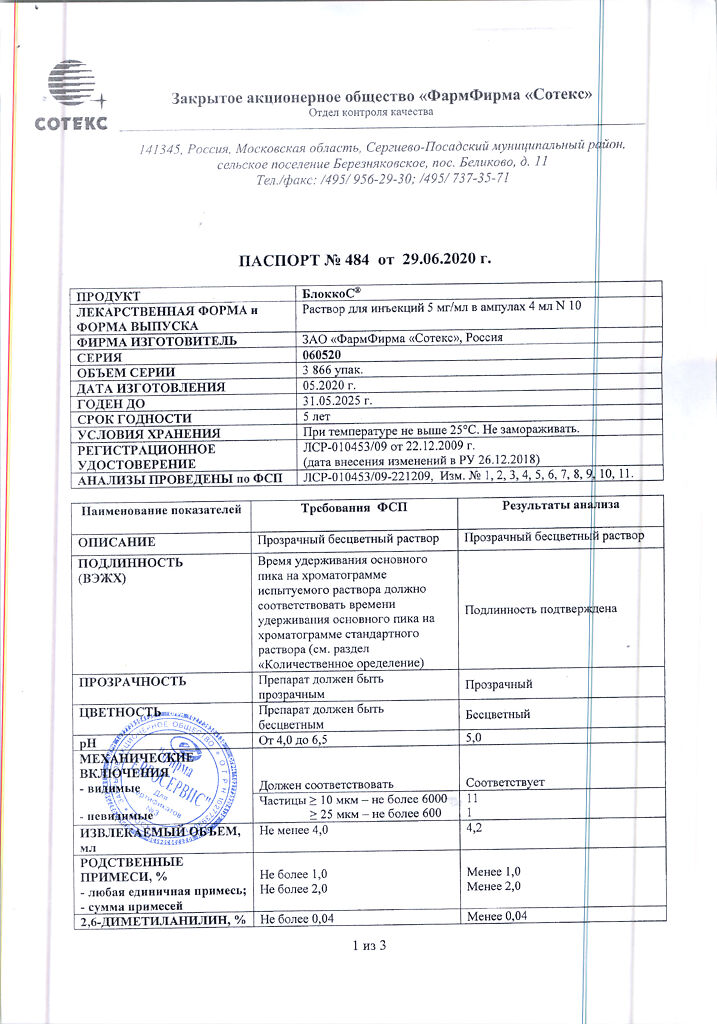
BlokkoS, 5 mg/ml 4 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
EAN: 4605964002914
SKU: 224240
Categories: Anesthesia and resuscitation, Local anesthetics, Medicine
Description
Pharmacodynamics:
Bupivacaine is a long-acting local anesthetic of the amide type. It reversibly blocks impulse conduction along the nerve fiber by disrupting the transport of sodium ions through sodium channels. Can have a similar effect in the brain and myocardium.
The most characteristic feature of bupivacaine is the duration of its action, which does not depend much on the addition of epinephrine to it. Bupivacaine is the drug of choice for continuous epidural anesthesia. In low concentrations it has less effect on motor fibers and has a shorter duration of action, which is useful for short-term pain relief, for example, during childbirth or after surgery.
The relative density of the drug solution is 1004 at 20 °С (which is equivalent to 1000 at 37 °С, the force of gravity has little effect on its distribution in the subarachnoid space. For subarachnoid administration, a small dose is administered, resulting in a relatively low concentration and a short duration of blockade. With subarachnoid administration of bupivacaine not containing dextrose, anesthesia is less predictable but more prolonged than with bupivacaine solution containing dextrose.
Pharmacokinetics:
The pKa value of bupivacaine is 8.2; the partition coefficient is 346 (at 25°C in n-octanol/phosphate buffer medium pH 7.4).
The absorption rate depends on the dose, the route of administration and the blood supply at the injection site. In intercostal blockade due to rapid absorption, the maximum plasma concentration is 4 mg/l (when administered 400 mg); plasma concentrations are lower in subcutaneous injections into the abdominal region. In children with caudal blockade, there is rapid absorption and high plasma concentrations of about 1-1.5 mg/l (when administered 3 mg/kg) are achieved.
Bupivacaine is completely absorbed from the epidural space, the half-life is biphasic and is 7 min and 6 h, respectively. Slow absorption limits the elimination rate of bupivacaine, which explains the longer elimination half-life after epidural injection than when administered intravenously.
The equilibrium volume of distribution of bupivacaine is 73 L, the hepatic extraction coefficient is 0.40, total plasma clearance is 0.58 L/min, and the plasma elimination half-life is 2.7 h. The half-life in infants may be longer than in adults, up to 8 hours. In children older than 3 months the half-life is equal to that of adults.
The binding to plasma proteins is 96%, mainly by α1-acid glycoprotein. After major surgery, the concentration of this protein may be elevated, which may result in a higher total plasma concentration of bupivacaine. The free fraction of bupivacaine is unchanged. Therefore, the potentially toxic plasma concentration is well tolerated.
Bupivacaine is almost completely metabolized in the liver, mainly by aromatic hydroxylation to 4-hydroxybupivacaine and N-dealkylation to pipecolyloxylidine, both reactions catalyzed by CYP3A4 isoenzyme. Thus, clearance depends on hepatic blood flow and the activity of metabolizing enzymes.
Bupivacaine penetrates through the placenta, the concentration of unbound bupivacaine in the fetus is equal to that of the mother. Because of the lower binding to plasma proteins, the fetal total plasma concentration is lower.
In intrathecal administration
Bupivacaine is well soluble in lipids with an oil/water distribution ratio of 27.5.
Bupivacaine is completely absorbed from the subarachnoid space in two phases with a half-life of 50-400 minutes. Slow absorption is a limiting factor in the excretion of bupivacaine, which explains its longer half-life than with intravenous administration.
The absorption from the subarachnoid space is relatively slow, which, combined with the low dose required for spinal anesthesia, results in a relatively low maximum plasma concentration (0.4 mg/mL per 100 mg of the drug).
Indications
Indications
Surgical anesthesia in adults and children over 12 years of age.
Acute pain in adults and children over 1 year of age.
Infiltration anesthesia, when it is necessary to achieve a long-term anesthetic effect, for example, for postoperative pain.
Long-acting conduction anesthesia or epidural anesthesia in cases in which the addition of epinephrine is contraindicated and significant muscle relaxation is undesirable. Anesthesia in obstetrics.
With intrathecal administration
Spinal anesthesia for surgical operations on the lower extremities, including operations on the hip joint, lasting 3–4 hours and not requiring significant motor block.
Pharmacological effect
Pharmacological effect
Pharmacodynamics:
Bupivacaine is a long-acting local anesthetic of the amide type. Reversibly blocks the conduction of impulses along the nerve fiber, disrupting the transport of sodium ions through sodium channels. May have a similar effect in the brain and myocardium.
The most characteristic feature of bupivacaine is its duration of action, which is not greatly affected by the addition of epinephrine. Bupivacaine is the drug of choice for continuous epidural anesthesia. At low concentrations, it has less effect on motor fibers and has a shorter duration of action, which is useful for short-term pain relief, for example, during childbirth or after surgery.
The relative density of the drug solution is 1004 at 20 ° C (equivalent to 1000 at 37 ° C), gravity has little effect on its distribution in the subarachnoid space. With subarachnoid administration, a small dose is administered, which leads to a relatively low concentration and short duration of blockade. With subarachnoid administration of bupivacaine, which does not contain dextrose, anesthesia is less predictable, but more longer than with the administration of a solution of bupivacaine containing dextrose.
Pharmacokinetics:
The pKa of bupivacaine is 8.2; separation coefficient – 346 (at 25 °C in n-octanol/phosphate buffer pH 7.4).
The rate of absorption depends on the dose, route of administration and blood supply at the injection site. With intercostal blockade, due to rapid absorption, the maximum plasma concentration is 4 mg/l (with the introduction of 400 mg); with subcutaneous injections into the abdominal area, plasma concentrations are lower. In children, with caudal blockade, rapid absorption occurs and a high plasma concentration is achieved – about 1-1.5 mg/l (with the introduction of 3 mg/kg).
Bupivacaine is completely absorbed from the epidural space, the half-life is biphasic and is 7 minutes and 6 hours, respectively. Slow absorption limits the rate of elimination of bupivacaine, which explains the longer half-life after administration into the epidural space than after intravenous administration.
The steady-state volume of distribution of bupivacaine is 73 L, the hepatic extraction coefficient is 0.40, the total plasma clearance is 0.58 L/min, and the plasma half-life is 2.7 hours. The half-life in newborns compared to adults can be longer up to 8 hours. In children older than 3 months, the half-life is equal to that of adults.
Plasma protein binding is 96%, predominantly with α1-acid glycoprotein. After major surgery, the concentration of this protein may be increased, which may result in higher total plasma concentrations of bupivacaine. The free fraction of bupivacaine does not change. Therefore, potentially toxic plasma concentrations are well tolerated.
Bupivacaine is almost completely metabolized in the liver, mainly by aromatic hydroxylation to 4-hydroxybupivacaine and N-dealkylation to pipecolylxylidine, both reactions catalyzed by the CYP3A4 isoenzyme. Thus, clearance depends on hepatic blood flow and the activity of metabolizing enzymes.
Bupivacaine crosses the placenta; the concentration of unbound bupivacaine in the fetus is equal to that of the mother. Due to lower plasma protein binding, total plasma concentrations are lower in the fetus.
With intrathecal administration
Bupivacaine is highly soluble in lipids with an oil-water partition coefficient of 27.5.
Bupivacaine is completely absorbed from the subarachnoid space in two phases with a half-life of 50–400 minutes. Slow absorption is the limiting factor for the elimination of bupivacaine, which explains its longer half-life than when administered intravenously.
Absorption from the subarachnoid space occurs relatively slowly, which, combined with the low dose required for spinal anesthesia, results in a relatively low maximum plasma concentration (0.4 mg/ml per 100 mg of drug).
Special instructions
Special instructions
The administration of local anesthetics must be carried out in the immediate vicinity of resuscitation equipment. Before starting large blockades, it is necessary to ensure venous access.
There have been reports of cardiac arrest or death during the use of bupivacaine for epidural anesthesia or peripheral blockade. In some cases, resuscitation was difficult or impossible despite adequate treatment.
Major peripheral nerve blocks may require large volumes of local anesthetic to be injected into well-perfused areas of the body, often near major blood vessels. In such situations, there is an increased risk of intravascular administration of bupivacaine or systemic absorption, which can lead to high concentrations of local anesthetic in the blood.
Like other local anesthetics, bupivacaine can cause acute toxic reactions in the central nervous and cardiovascular systems if high concentrations in the blood occur during the local anesthetic procedure. This most often occurs in the case of inadvertent intravascular injection or when there is a good blood supply to the injection site.
Certain types of local anesthesia, regardless of the local anesthetic used, can cause serious adverse reactions, for example:
· Epidural anesthesia, especially against the background of hypovolemia, can lead to depression of the cardiovascular system. Caution should be exercised in patients with cardiovascular diseases.
· When administered retrobulbarically, the drug may occasionally penetrate into the cranial subarachnoid space, causing temporary blindness, apnea, convulsions, collapse and other adverse reactions. Relief of these reactions must be carried out immediately.
· With retrobulbar and peribulbar administration of local anesthetics, there is a certain risk of persistent dysfunction of the eye muscles. The main causes are trauma and/or local toxicity to muscles and/or nerves.
· If inadvertently administered intravascularly to the head and neck area, even at low doses, it can cause cerebral symptoms.
The severity of the adverse reactions described above depends on the size of the injury, the concentration of the local anesthetic and the duration of its effect on the tissue. Therefore, as with other local anesthetics, the lowest effective dose of bupivacaine should be administered.
Caution must be exercised in patients with second or third degree AV block, as local anesthetics may impair intracardiac conduction. Caution should be exercised when administering the drug to the elderly and to patients with severe liver disease, severe renal failure or general poor condition.
Careful monitoring and continuous ECG monitoring should be carried out in patients receiving class III antiarrhythmic drugs (for example, amiodarone), since their cardiovascular toxic effects may be additive with those of bupivacaine.
During epidural anesthesia, a decrease in blood pressure and bradycardia may occur. The likelihood of such complications can be reduced by prior administration of crystalloid and colloid solutions. If blood pressure decreases, immediate intravenous administration of sympathomimetics (for example, 5–10 mg ephedrine) is indicated; if necessary, their administration is repeated.
According to post-registration surveillance data, chondrolysis occurred in some patients who received local anesthetics into the joint cavity after long-term surgery. In most cases, chondrolysis of the shoulder joint. A cause-and-effect relationship with the administration of bupivacaine has not been conclusively confirmed; chondrolysis may be due to several factors. The literature describes conflicting data on the mechanism of occurrence of this condition. Long-term intra-articular administration is not an approved indication for bupivacaine.
The solution does not contain preservatives, so it must be administered immediately after opening the bottle. The remaining solution must be destroyed.
Children
The safety and effectiveness of bupivacaine in children less than 1 year of age have not been studied and only limited data are available.
There are no data on intra-articular blockade with bupivacaine in children 1–12 years of age.
There are no data on large nerve blockade with bupivacaine in children 1–12 years of age.
During epidural anesthesia, the drug should be administered slowly, based on age and body weight, since severe hypotension and respiratory distress may occur, especially with epidural anesthesia at chest level.
With intrathecal administration
Spinal anesthesia can cause severe blockages and paralysis of the intercostal muscles and diaphragm, especially in pregnant women.
Elderly patients and patients in late pregnancy have an increased risk of high or complete spinal block, leading to circulatory and respiratory depression. In these patients the dose should be reduced.
Spinal anesthesia may result in decreased blood pressure and bradycardia. This risk can be reduced by administration of crystalloid solutions. If blood pressure decreases, it must be stopped immediately, for example, by intravenous administration of 5–15 mg of ephedrine.
Patients with hypovolemia, regardless of its cause, may develop a sudden severe decrease in blood pressure during intrathecal anesthesia.
Spinal anesthesia may cause intercostal paralysis, and therefore, patients with pleural effusion may experience respiratory failure. Septicemia may increase the risk of intraspinal abscess formation in the postoperative period.
Neurological complications are a rare consequence of spinal anesthesia and can lead to paresthesia, anesthesia, muscle weakness and paralysis. In some cases, these complications are permanent.
Before starting treatment, the benefits and risks for the patient should be weighed.
Impact on the ability to drive vehicles and operate machinery
Depending on the dose and route of administration, local anesthetics may have a transient effect on motor function and coordination.
Active ingredient
Active ingredient
Bupivacaine
Composition
Composition
1 ampoule (4 ml) contains
as an active substance
bupivacaine hydrochloride monohydrate in terms of dry matter 20 mg;
Excipients:
sodium chloride 32.0 mg,
disodium edetate 0.4 mg,
hydrochloric acid solution 0.1 M or sodium hydroxide solution 0.1 M to pH 4.0-6.5,
water for injections up to 4 ml.
Pregnancy
Pregnancy
With paracervical blockade in obstetrics, bupivacaine can cause severe cardiovascular disorders in the fetus. The use of bupivacaine as a paracervical blockade is contraindicated (see section “Contraindications”).
The use of the drug according to the stated indications for use is possible if the expected benefit to the mother outweighs the possible risk to the fetus.
Bupivacaine passes into breast milk, but when used in therapeutic doses, the effect on the child is negligible.
With intrathecal administration
There are no data on undesirable effects on pregnancy. When used in pregnant animals in large doses, a decrease in the survival rate of offspring in rats and embryological effects in rabbits were found. Therefore, bupivacaine should not be used in early pregnancy unless the benefits outweigh the risks.
In late pregnancy, the dose should be reduced (see section “Special Instructions”). Bupivacaine passes into breast milk, but when used in therapeutic doses, the effect on the child is negligible.
Contraindications
Contraindications
Hypersensitivity to any of the components of the drug or to other amide-type local anesthetics.
Severe arterial hypotension (cardiogenic or hypovolemic shock).
Children under 1 year of age – for all indications for use, with the exception of intrathecal anesthesia, in which the drug can be administered from birth.
Intravenous regional anesthesia (Beer block) (accidental penetration of bupivacaine into the bloodstream can cause the development of acute systemic toxic reactions).
Paracervical blockade in obstetrics.
Conditions that are contraindications to epidural or intrathecal anesthesia:
Diseases of the central nervous system (for example, meningitis, tumors, polio, cranial bleeding).
Purulent skin infections at or near the site of a lumbar puncture.
Spinal stenosis, active disease (eg, spondylitis, tumors, tuberculosis) or trauma (eg, fracture) of the spine.
Sepsis, subacute spinal cord degeneration associated with megaloblastic anemia.
Cardiogenic or hypovolemic shock.
Coagulation disorders or active anticoagulant therapy.
With caution
Cardiovascular failure (possible progression), heart block, inflammatory diseases or infection of the injection site (during infiltration anesthesia), cholinesterase deficiency, renal failure, old age (over 65 years), late pregnancy (III trimester, see sections “Use during pregnancy and breastfeeding” and “Special instructions”), general severe condition, decreased hepatic blood flow (for example, in chronic heart failure, liver disease), simultaneous administration of antiarrhythmic drugs (including β-blockers), children’s age (1–12 years).
Bupivacaine should be used with caution in patients receiving other local anesthetics or drugs structurally similar to amide-type local anesthetics, such as antiarrhythmic drugs (eg, lidocaine, mexiletine).
Side Effects
Side Effects
Adverse drug reactions caused by the drug may be difficult to distinguish from physiological manifestations of nerve blockade (eg, decreased blood pressure, bradycardia), reactions directly (eg, nerve damage) or indirectly caused by administration (eg, epidural abscess).
Neurological disorders are a rare but well-known adverse drug reaction associated with local anesthesia, especially with epidural and intrathecal drug administration.
Symptoms and management of acute systemic toxicity are described in the Overdose section.
Organ system
Frequency
Adverse drug reaction
From the immune system
Rare (≥1/10,000, <1/1000)
Allergic reactions, anaphylactic shock
From the central and peripheral nervous system
Often (≥1/100, <1/10)
Paresthesia, dizziness
Uncommon (≥1/1000, <1/100)
Signs of toxicity from the central nervous system (convulsions, paresthesia in the mouth, numbness of the tongue, hyperacusis, visual disturbances, loss of consciousness, tremor, dizziness, tinnitus, dysarthria)
Rare (≥1/10,000, <1/1000)
Neuropathy, peripheral nerve damage, arachnoiditis, paresis, paraplegia
From the side of the organ of vision
Rare (≥1/10,000, <1/1000)
Diplopia
From the side of the heart
Often (≥1/100, <1/10)
Bradycardia
Rare (≥1/10,000, <1/1000)
Cardiac arrest, arrhythmia
From the side of blood vessels
Very common (≥1/10)
Lower blood pressure
Often (≥1/100, <1/10)
Increased blood pressure
From the respiratory system, chest and mediastinum
Rare (≥1/10,000, <1/1000)
Respiratory depression
From the gastrointestinal tract
Very common (≥1/10)
Nausea
Often (≥1/100, <1/10)
Vomit
From the kidneys and urinary tract
Often (≥1/100, <1/10)
Urinary retention
Adverse reactions in children are similar to those in adults, however, early signs of local anesthetic toxicity in children may be more difficult to recognize if the block is performed under sedation or anesthesia.
With intrathecal administration
Very often
(>1/10)
From the side of the heart:
Decreased blood pressure, bradycardia
From the gastrointestinal tract:
Nausea
Often
(>1/100, <1/10)
From the nervous system:
Headache after dural puncture
From the gastrointestinal tract:
Vomit
From the kidneys and urinary tract:
Urinary retention, urinary incontinence
Uncommon
(>1/1000, <1/100)
From the nervous system:
Paresthesia, paresis, dysesthesia
Sides of skeletal muscle, connective tissue and bone:
Muscle weakness, back pain
Rarely
(< 1/1000)
From the side of the heart:
Heart failure
From the immune system:
Allergic reactions, anaphylactic shock
From the nervous system:
Complete unintentional spinal block, paraplegia, paralysis, neuropathy, arachnoiditis
From the side of breathing
Respiratory depression
Adverse reactions in children are similar to those in adults, however, early signs of local anesthetic toxicity in children may be more difficult to recognize if the block is performed under sedation or anesthesia.
Interaction
Interaction
Caution should be exercised when using bupivacaine concomitantly with other local anesthetics or class Ib antiarrhythmics, as they may enhance each other’s toxic effects.
A separate study of the interaction between local anesthetics and class III antiarrhythmic drugs (for example, amiodarone) has not been conducted; however, caution is recommended when using them simultaneously (see also section “Special instructions”).
Alkalinization may lead to precipitation as the solubility of bupivacaine decreases at pH > 6.5.
When preparing for administration, it is necessary to avoid prolonged contact of the drug with metal objects, since metal ions can cause reactions at the injection site, manifested by pain and swelling.
When adding epinephrine to a local anesthetic solution, it is necessary, if possible, to avoid their simultaneous use with monoamine oxidase inhibitors and tricyclic antidepressants, since a persistent increase in blood pressure may develop. If such simultaneous therapy is necessary, the patient must be closely monitored. Concomitant use with vasopressor and uterotonic agents (ergot derivatives) can lead to a persistent increase in blood pressure and cerebrovascular complications. Phenothiazine and butyrophenone derivatives can reduce or reverse the pressor effect of epinephrine.
Overdose
Overdose
Acute systemic toxicity
Symptoms
Toxic reactions manifest themselves mainly in the central nervous and cardiovascular systems. They occur due to high concentrations of local anesthetic in the blood, which may be due to accidental intravascular administration, overdose, or extremely rapid absorption from highly vascularized tissues (see section “Special Instructions”).
Signs of damage to the central nervous system for all amide-type local anesthetics are similar to each other, while symptoms of damage to the cardiovascular system differ, both qualitatively and quantitatively.
Inadvertent intravascular administration of local anesthetic may result in immediate systemic toxic reactions (within seconds to minutes). Signs of systemic toxicity in case of overdose appear later (15–60 minutes after administration), since the concentration of local anesthetic in the blood increases slowly.
Intoxication of the central nervous system manifests itself gradually, the severity of symptoms increases gradually. Initial signs are usually: dizziness, paresthesia around the mouth, numbness of the tongue, hyperacusis, tinnitus and visual disturbances. More serious manifestations are dysarthria and myofasciculations, which may precede the onset of generalized seizures. These phenomena should not be mistaken for a neurotic disorder. They may be followed by loss of consciousness and the development of a large convulsive seizure lasting from several seconds to several minutes. Due to increased muscle activity and insufficient ventilation during convulsions, hypoxia and hypercapnia quickly increase. In severe cases, respiratory arrest may occur. Concomitant acidosis aggravates the toxic effect of local anesthetics.
Resolution of symptoms occurs due to the metabolism of the local anesthetic and its redistribution from the central nervous system. The described phenomena quickly stop if the overdose was not excessive.
Damage to the cardiovascular system, as a rule, indicates more severe intoxication. It is usually preceded by signs of central nervous system damage, which can be erased if the patient is under anesthesia or deep sedation caused by drugs such as benzodiazepines or barbiturates. Due to the high concentration of local anesthetics in the blood, a decrease in blood pressure, bradycardia, arrhythmias and cardiac arrest may occur. Toxic manifestations from the cardiovascular system are often caused by myocardial depression and conduction disturbances, causing decreased cardiac output, decreased blood pressure, AV block, bradycardia, ventricular arrhythmia, including ventricular tachycardia and ventricular fibrillation, and cardiac arrest. These events are often preceded by severe signs of central nervous system damage, including seizures, but in rare cases, cardiac arrest occurs without associated central nervous system symptoms. After a very rapid intravenous injection, the concentration of bupivacaine in the blood can be quite high. In this case, it quickly reaches the coronary arteries, and symptoms of circulatory disorders occur before signs of central nervous system damage. This mechanism causes myocardial depression and may serve as the first manifestation of intoxication.
When performing a blockade under general anesthesia in children, early signs of intoxication are difficult to detect, and therefore careful monitoring is required.
Treatment
If a spinal block occurs, it is necessary to ensure adequate ventilation (ensure airway patency, oxygen supply, and, if necessary, perform intubation and artificial ventilation). If blood pressure decreases and (or) bradycardia, a vasopressor with inotropic action must be administered.
If symptoms of acute systemic intoxication occur, administration of the drug should be stopped immediately. Proper ventilation, oxygenation and circulation must be maintained.
In all cases, it is necessary to establish an oxygen supply; if necessary, intubation and controlled ventilation (in some cases with hyperventilation) are carried out. For convulsions, diazepam is administered, and for bradycardia, atropine is administered. In case of circulatory failure – dobutamine intravenously, norepinephrine can be administered (starting from 0.05 mcg/kg/min, if necessary, increasing the dose by 0.05 mcg/kg/min every 10 minutes); in more severe cases, the dose is titrated based on the results of hemodynamic monitoring. It is possible to administer ephedrine. In case of severe damage to the cardiovascular system, resuscitation measures can continue for several hours. Any acidosis must be eliminated.
When relieving systemic intoxication in children, doses of medications must be selected in accordance with their age and body weight.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C (do not freeze)
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
PharmFirma Sotex, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C (do not freeze) |
| Manufacturer | PharmFirm Sotex, Russia |
| Medication form | solution for injection |
| Brand | PharmFirm Sotex |
Related products
Buy BlokkoS, 5 mg/ml 4 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.