No products in the cart.
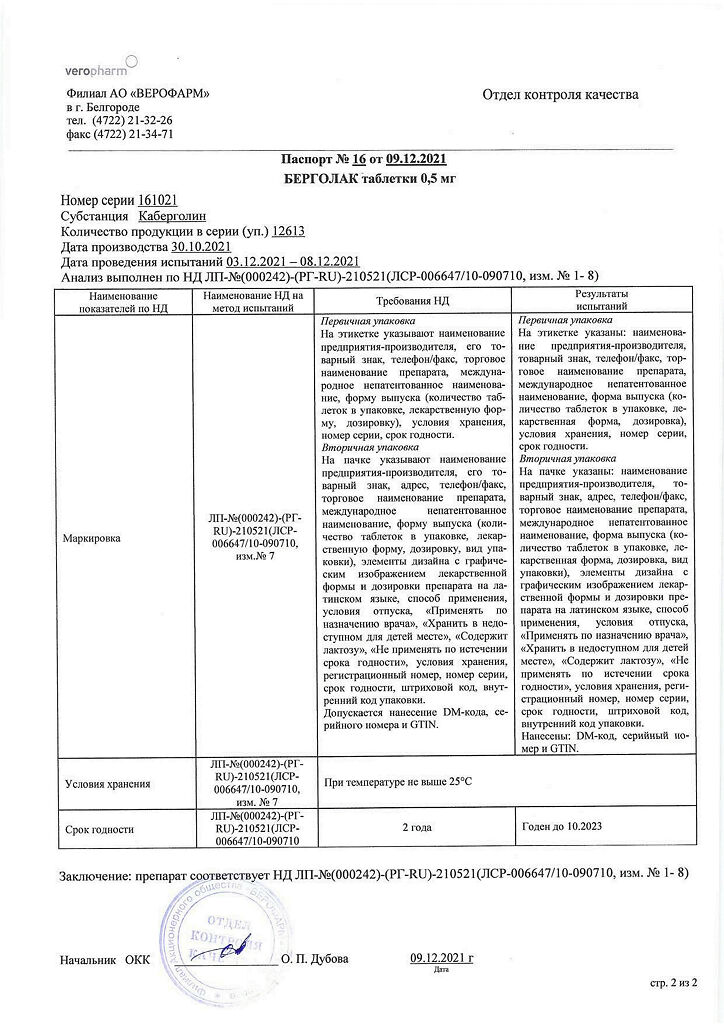
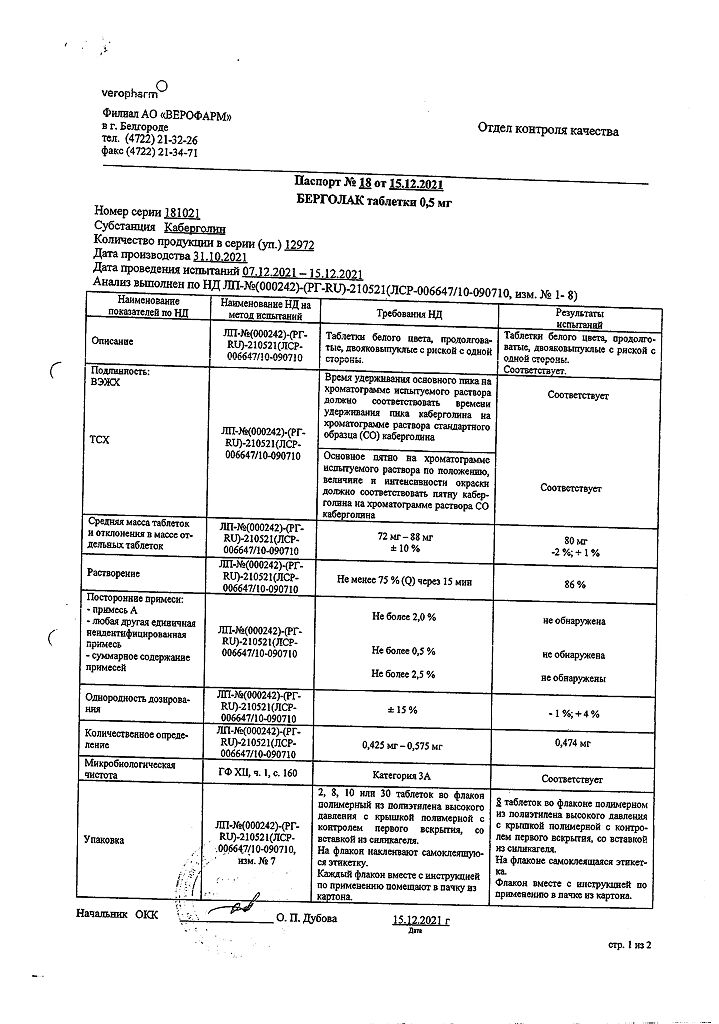
Bergolak, tablets 0.5 mg 8 pcs
€34.58 €28.81
Out of stock
(E-mail when Stock is available)
Description
Dopamine receptor agonist, ergoline derivative, inhibits prolactin secretion. Stimulates dopamine D2 receptors of lactotropic pituitary cells; in high doses has a central dopaminergic effect. Reduces the concentration of prolactin in the blood, restores the menstrual cycle and fertility.
. By reducing the concentration of prolactin in blood in women, pulsatile gonadotropin secretion and release of luteinizing hormone in the middle of the cycle is restored, anovulatory cycles are eliminated and the concentration of estrogen in the body is increased; the severity of hypoestrogenic (weight gain, fluid retention, osteoporosis) and hyperandrogenic (acne, hirsutism) symptoms is reduced.
In men it decreases libido and impotence caused by hyperprolactinemia (when the concentration of prolactin decreases, the concentration of testosterone increases), gynecomastia and lactorrhea.
Pituitary macroadenomas and related symptoms (headache, disorders of visual fields and visual acuity, cranial nerve functions and anterior pituitary lobe) are reversed. Decreases the concentration of prolactin in patients with prolactinoma and pseudoprolactinoma (in the latter – without reducing the size of pituitary adenoma). Decrease of prolactin concentration is noticed in 3 hours after treatment and lasts for 7-28 days in patients with hyperprolactinemia and 14-21 days – when we suppress postpartum lactation. Decrease of prolactin concentration occurs within 2-4 weeks of treatment.
Pharmacokinetics
Absorption and distribution
Absorption is high, not dependent on food intake. Time to reach Cmax in blood plasma is 0.5-4 hours. Binding to plasma proteins is 41-42%.
T1/2 is 63-68 h in healthy volunteers and 79-115 h in patients with hyperprolactinemia. Due to the long T1/2 equilibrium concentration state is reached after 4 weeks of therapy.
Metabolism and excretion
Actively metabolized. Metabolites have significantly less effect in suppressing prolactin secretion compared to cabergoline.
The urinary and intestinal excretion is about 18% and 72% of the ingested dose of cabergoline, respectively, with 2-3% excretion of unchanged cabergoline in the kidneys.
Indications
Indications
prevention of postpartum lactation;
suppression of already established postpartum lactation;
treatment of disorders associated with hyperprolactinemia, including amenorrhea, oligomenorrhea, anovulation, galactorrhea;
prolactin-secreting pituitary adenomas (micro- and macroprolactinomas); idiopathic hyperprolactinemia; empty sella syndrome in combination with hyperprolactinemia.
Pharmacological effect
Pharmacological effect
A dopamine receptor agonist, ergoline derivative, suppresses prolactin secretion. Stimulates dopamine D2 receptors of lactotropic cells of the pituitary gland; in high doses it has a central dopaminergic effect. Reduces the concentration of prolactin in the blood, restores the menstrual cycle and fertility.
By reducing the concentration of prolactin in the blood of women, the pulsating secretion of gonadotropins and the release of luteinizing hormone in the middle of the cycle are restored, anovulatory cycles are eliminated and the concentration of estrogen in the body increases, the severity of hypoestrogenic (weight gain, fluid retention, osteoporosis) and hyperandrogenic (acne, hirsutism) symptoms is reduced.
In men, it reduces the decrease in libido caused by hyperprolactinemia, impotence (with a decrease in the concentration of prolactin, the concentration of testosterone increases), gynecomastia, and lactorrhea.
Pituitary macroadenomas and associated symptoms (headache, impaired visual fields and acuity, functions of the cranial nerves and the anterior pituitary gland) are subject to reverse development. Reduces the concentration of prolactin in patients with prolactinoma and pseudoprolactinoma (in the latter, without reducing the size of the pituitary adenoma). A decrease in prolactin concentration is observed 3 hours after administration and persists for 7-28 days in patients with hyperprolactinemia and 14-21 days in cases of suppressed postpartum lactation. A decrease in prolactin concentration occurs within 2-4 weeks of treatment.
Pharmacokinetics
Suction and distribution
Absorption is high and does not depend on food intake. Time to reach Cmax in blood plasma is 0.5-4 hours. Plasma protein binding is 41-42%.
T1/2 is 63-68 hours in healthy volunteers and 79-115 hours in patients with hyperprolactinemia. Due to the long T1/2, a state of equilibrium concentration is achieved after 4 weeks of therapy.
Metabolism and excretion
Actively metabolized. Metabolites have a significantly lesser effect in suppressing prolactin secretion compared to cabergoline.
About 18% and 72% of the dose of cabergoline taken is excreted by the kidneys and through the intestines, respectively, while 2-3% is excreted by the kidneys as unchanged cabergoline.
Special instructions
Special instructions
Before prescribing cabergoline, a complete study of pituitary function should be performed.
When increasing the dose of the drug, patients should be under medical supervision in order to establish the lowest dose that provides a therapeutic effect. During the treatment period, it is recommended to regularly (once a month) determine the concentration of prolactin in the blood serum. Normalization of prolactin concentrations is usually observed within 2-4 weeks of cabergoline therapy.
After discontinuation of cabergoline, a relapse of hyperprolactinemia is usually observed, but in some patients a persistent decrease in prolactin concentration persists for several months. Most women continue to have ovulatory cycles for at least 6 months after stopping cabergoline.
Cabergoline restores ovulation and fertility in women with hyperprolactinemic hypogonadism. Since pregnancy may occur before menstruation returns, it is recommended that pregnancy tests be performed at least once every 4 weeks during the amenorrhea period, and after menstruation returns, whenever menstruation is more than 3 days late.
Barrier methods of contraception should be used during treatment with cabergoline, as well as after discontinuation of the drug until anovulation recurs. If pregnancy occurs during treatment, the advisability of discontinuing the drug should be considered. Women who become pregnant should be under medical supervision to promptly identify symptoms of an enlarged pituitary gland (during pregnancy, pre-existing pituitary tumors may increase in size).
After long-term use of cabergoline, pleural effusion/pleural fibrosis and valvulopathy were observed in patients, therefore cabergoline should be used with caution in patients with current manifestations and/or clinical symptoms of cardiac dysfunction, incl. in the anamnesis.
In patients with hypertension that developed during pregnancy (for example, preeclampsia) and/or postpartum hypertension, cabergoline is prescribed only in cases where the potential benefit of the drug significantly outweighs the possible risk.
The use of cabergoline causes drowsiness. In patients with Parkinson’s disease, the use of dopamine receptor agonists may cause sudden sleep onset. In such cases, it is recommended to reduce the dose of cabergoline or discontinue therapy.
No studies have been conducted on the use of cabergoline in elderly patients with disorders associated with hyperprolactinemia.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, it is recommended to refrain from driving vehicles and performing other activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Cabergoline
Composition
Composition
1 tablet contains:
cabergoline 500 mcg
Excipients:
leucine,
lactose anhydrous (lactopress),
magnesium stearate.
Pregnancy
Pregnancy
Since controlled clinical studies with the use of cabergoline in pregnant women have not been conducted, the use of the drug during pregnancy is possible only in cases of extreme necessity, when the potential benefit of the drug for the woman significantly outweighs the possible risk to the fetus.
Pregnancy should be avoided for at least one month after discontinuation of cabergoline, given the long half-life of the drug and the limited data on its effects on the fetus (according to available data, the use of cabergoline at a dose of 0.5-2 mg per week for disorders associated with hyperprolactinemia was not accompanied by an increase in the incidence of miscarriages, premature births, multiple pregnancies and congenital malformations).
If pregnancy occurs during treatment with cabergoline, the advisability of discontinuing the drug should be considered, also taking into account the ratio of the potential benefits of using the drug for the woman and the possible risk to the fetus.
Since cabergoline suppresses lactation, the drug should not be prescribed to mothers who wish to breastfeed. Breastfeeding should be discontinued during treatment with cabergoline.
Use in children
Contraindicated in patients under 16 years of age (safety and effectiveness in this category of patients have not been established).
Contraindications
Contraindications
age up to 16 years;
rare hereditary forms of galactose intolerance, congenital lactase deficiency lapp or impaired absorption of glucose-galactose (since the drug contains lactose);
hypersensitivity to cabergoline or other components of the drug, as well as to any ergot alkaloids.
The drug should be prescribed with caution for the following conditions and/or diseases:
arterial hypertension that developed during pregnancy (for example, preeclampsia) and/or postpartum arterial hypertension;
severe diseases of the cardiovascular system, Raynaud’s syndrome;
peptic ulcer, gastrointestinal bleeding;
severe liver failure;
severe psychotic and cognitive disorders (including history);
the presence of fibrotic changes in the heart (valvulopathy) and respiratory system (pleurisy/pleural fibrosis), incl. in the anamnesis;
simultaneous use with drugs that have a hypotensive effect (due to the risk of developing orthostatic hypotension).
Side Effects
Side Effects
Side effects are usually transient, but the severity is mild or moderate and is dose-dependent. They occur mainly during the first 2 weeks of therapy and in most cases disappear on their own as therapy continues or a few days after cabergoline is discontinued.
From the cardiovascular system: palpitations, flushes of blood to the skin of the face, spasms of blood vessels in the fingers (like other ergot derivatives, cabergoline can have a vasoconstrictor effect), valvulopathy; rarely – orthostatic hypotension (with long-term treatment with cabergoline – a hypotensive effect), an asymptomatic decrease in blood pressure was observed during the first 3-4 days after birth (systolic – by more than 20 mm Hg, diastolic – by more than 10 mm Hg).
From the nervous system: dizziness/vertigo, headache, fatigue, drowsiness, depression, mania, asthenia, paresthesia, fainting.
From the digestive system: nausea, vomiting, pain in the epigastric region, abdominal pain, constipation, gastritis, dyspepsia, impaired liver function.
Allergic reactions: hypersensitivity reactions, skin rash.
Other: mastodynia, nosebleeds, transient hemianopsia, muscle cramps of the lower extremities, alopecia, increased CPK activity in the blood serum, edema, pleural fibrosis, respiratory disorders (including respiratory failure).
Interaction
Interaction
Concomitant use with ergot alkaloids and their derivatives is not recommended (with long-term therapy with cabergoline).
With simultaneous use, dopamine receptor antagonists (phenothiazine derivatives, butyrophenone, thioxanthene; metoclopramide) may weaken the effect of cabergoline.
The simultaneous use of cabergoline with macrolides is not recommended due to a possible increase in the concentration of cabergoline in the blood plasma. The mechanism of interaction between macrolides and cabergoline has not been sufficiently studied, but is apparently explained by the ability of macrolides and cabergoline to competitively inhibit the cytochrome P450 system.
Overdose
Overdose
Symptoms: nausea, vomiting, abdominal pain, constipation, decreased blood pressure, orthostatic hypotension, headache, calf muscle cramps, severe asthenia, sweating, drowsiness, psychomotor agitation, psychosis, hallucinations.
Treatment: gastric lavage, blood pressure control, administration of dopamine receptor antagonists (phenothiazine derivatives, butyrophenone, thioxanthene, metoclopramide).
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Veropharm LLC, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Veropharm AO, Russia |
| Medication form | pills |
| Brand | Veropharm AO |
Related products
Buy Bergolak, tablets 0.5 mg 8 pcs with delivery to USA, UK, Europe and over 120 other countries.