No products in the cart.
Baralgin M, tablets 500 mg 100 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Baralgin is antispasmodic, analgesic, antipyretic, anti-inflammatory.
Blocks cyclooxygenase and reduces GH synthesis.
Pharmacokinetics
. After oral administration, it is hydrolyzed in the gastrointestinal tract to form the active metabolite, 4-methylaminoantipyrine (4-MMA), which is absorbed in the liver to 4-aminoantipyrine (4-AA), as well as pharmacologically inactive metabolites.
After ingestion of 1 g of metamizole, 58% of 4-MMA and 48% of 4-AA are bound to plasma proteins.
The effective therapeutic plasma concentration of 4-MMA is reached after 20-40 min, Cmax after 2 h.
Indications
Indications
Severe acute or chronic pain syndrome due to injuries and postoperative pain syndrome, colic, cancer and other conditions where other therapeutic measures are contraindicated.
Fever resistant to other treatments.
Pharmacological effect
Pharmacological effect
Baralgin – antispasmodic, analgesic, antipyretic, anti-inflammatory.
Blocks cyclooxygenase and reduces PG synthesis.
Pharmacokinetics
After oral administration, it is hydrolyzed in the gastrointestinal tract to form an active metabolite – 4-methylaminoantipyrine (4-MMA), which is absorbed in the liver into 4-aminoantipyrine (4-AA), as well as pharmacologically inactive metabolites.
After oral administration of 1 g of metamizole, 58% of 4-MMA and 48% of 4-AA are bound to plasma proteins.
The effective therapeutic concentration in plasma of 4-MMA is achieved after 20–40 minutes, Cmax after 2 hours.
Special instructions
Special instructions
In patients suffering from bronchial asthma, taking the drug can provoke the development of an attack.
Active ingredient
Active ingredient
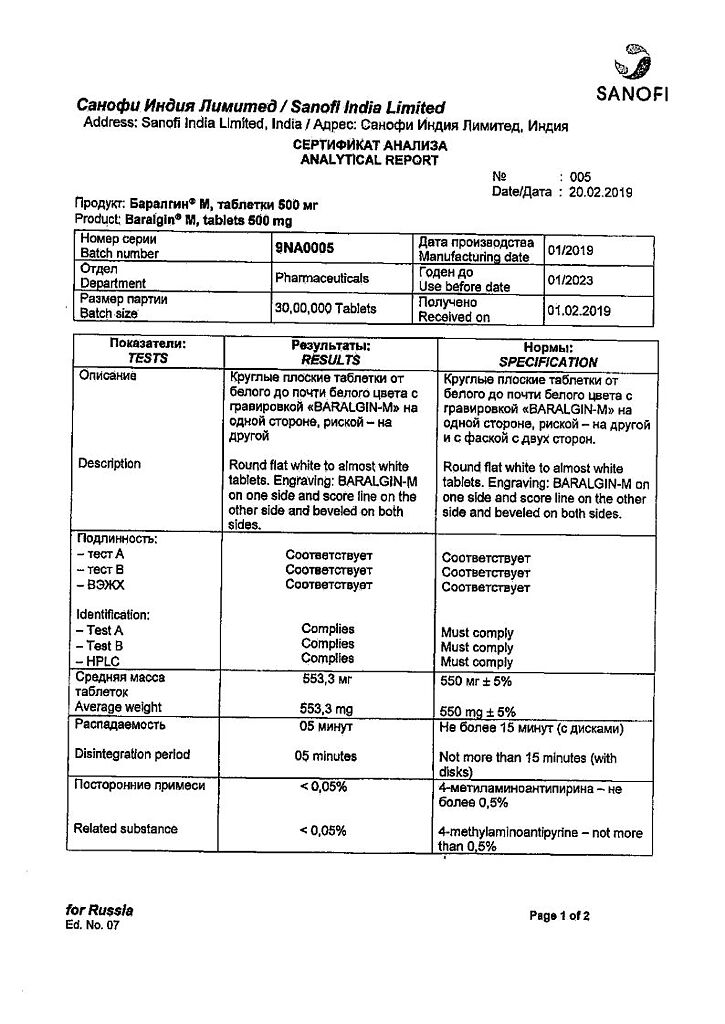
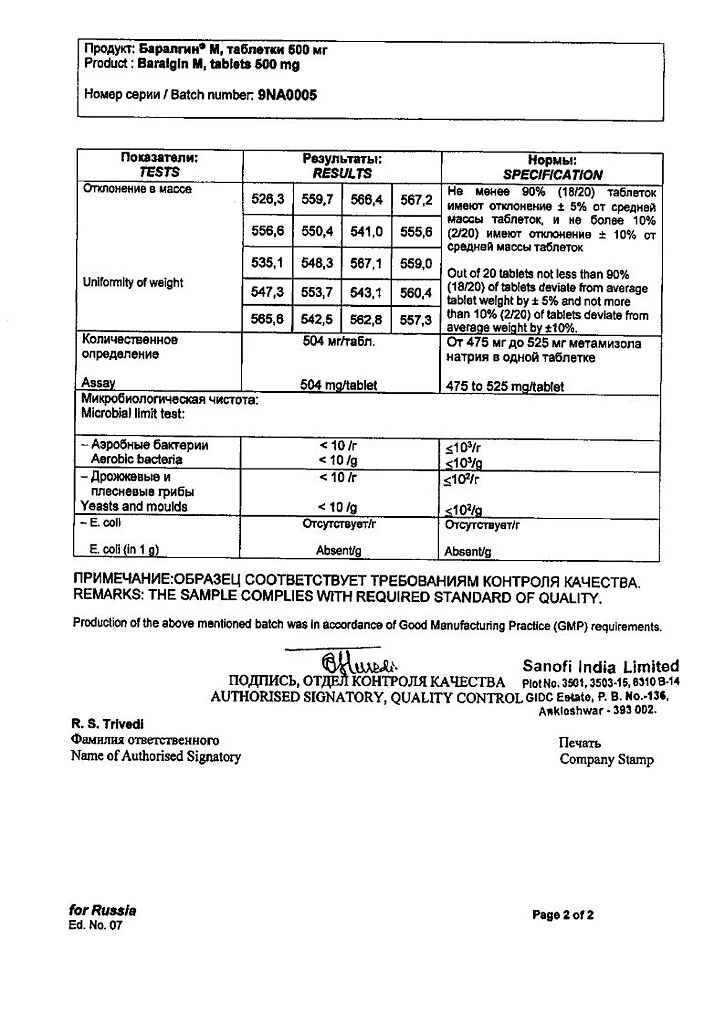
Metamizole sodium
Composition
Composition
Active ingredient:
metamizole sodium 500 mg;
Excipients:
macrogol 4000 47 mg,
magnesium stearate 3 mg.
Pregnancy
Pregnancy
Pregnancy
There is no sufficient clinical data on the use of metamizole sodium in pregnant women, therefore use during pregnancy is not recommended.
Breastfeeding period
Metabolites of metamizole sodium pass into breast milk, therefore, when using the drug, as well as within 48 hours after taking/administering the last dose, you must stop breastfeeding.
Contraindications
Contraindications
– Hypersensitivity to metamizole sodium and other components of the drug, as well as other pyrazolones (phenazone, propyphenazone) or pyrazolidines (phenylbutazone, oxyphenbutazone), including, for example, a history of agranulocytosis when taking one of these drugs.
– Disorders of bone marrow hematopoiesis (for example, after treatment with cytostatics) or diseases of the hematopoietic system.
– History of bronchospasm or other anaphylactic reactions (eg, urticaria, rhinitis, angioedema) when taking analgesic drugs such as salicylates, paracetamol, diclofenac, ibuprofen, indomethacin, naproxen.
– Congenital deficiency of glucose-6-phosphate dehydrogenase (risk of hemolysis).
– Children’s age (up to 15 years).
– Pregnancy (first and third trimester).
– Lactation period.
– Acute intermittent hepatic porphyria (risk of developing porphyria attacks).
If you have one of these diseases or conditions, consult your doctor before taking this medicine.
With caution:
– Hypotension (systolic blood pressure below 100 mm Hg), decreased circulating blood volume, hemodynamic instability (myocardial infarction, multiple trauma, incipient shock), incipient heart failure, high fever (increased risk of a sharp decrease in blood pressure).
– Diseases in which a significant decrease in blood pressure may be of increased danger (patients with severe coronary heart disease and stenosis of the cerebral arteries).
– Alcoholism.
– Bronchial asthma, especially in combination with concomitant polypous rhinosinusitis; chronic urticaria and other types of atopy (allergic diseases, in the development of which a hereditary predisposition to sensitization plays a significant role: hay fever, allergic rhinitis, etc.) (increased risk of developing anaphylactic/anaphylactoid reactions).
– Alcohol intolerance (reaction to even small amounts of certain alcoholic beverages with symptoms such as itching, watery eyes and severe redness of the face) (increased risk of developing anaphylactic/anaphylactoid reactions).
– Intolerance to dyes (eg tartrazine) or preservatives (eg benzoates) (increased risk of anaphylactic/anaphylactoid reactions).
– Severe liver and kidney dysfunction (low doses are recommended due to the possibility of slowing down the excretion of metamizole sodium).
– Pregnancy (second trimester).
Side Effects
Side Effects
Side effects were classified according to the recommendations of the World Health Organization: very common (≥10%); often (≥1%, <10%); uncommon (≥0.1%, <1%); rare (≥0.01%, <0.1%); very rare (<0.01%); frequency unknown (from the available data it is impossible to estimate the frequency of side effects).Cardiac disordersNot known: Kounis syndrome (allergic angina or allergic myocardial infarction).Immune system disordersRare: Metamizole sodium may cause anaphylactic or anaphylactoid reactions, which can be severe and life-threatening; In some cases, anaphylactic reactions can be fatal.In case of development of anaphylactic/anaphylactoid reactions, it is necessary to immediately stop taking the drug, take measures to provide emergency medical care to patients, and conduct a detailed clinical blood test.These reactions can occur even if the drug has been used many times before without any complications. These drug reactions may occur immediately or several hours after taking metamizole sodium, but they usually occur within one hour.Typically, milder anaphylactic or anaphylactoid reactions manifest themselves in the form of skin and mucosal symptoms (itching, burning, flushing, urticaria, swelling), shortness of breath or complaints from the gastrointestinal tract.Milder reactions may progress to severe forms with generalized urticaria, severe angioedema (especially involving the larynx), severe bronchospasm, cardiac arrhythmias, a sharp decrease in blood pressure (sometimes preceded by an increase in blood pressure) and the development of circulatory shock.Very rare: in patients with a complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses and intolerance to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (including a history), intolerance reactions usually manifest themselves in the form of attacks of bronchial asthma.Frequency unknown: anaphylactic shock.Disorders of the skin and subcutaneous tissuesUncommon: In addition to the manifestations of anaphylactic/anaphylactoid reactions on the skin and mucous membranes listed above, a fixed drug rash may infrequently occur.Rare: rash may occur.Very rare: possible development of Stevens-Johnson syndrome or Lyell’s syndrome (toxic epidermal necrolysis).Blood and lymphatic system disordersRare: leukopenia.Very rare: agranulocytosis (including fatal cases), thrombocygopenia.Not known: aplastic anemia, pancytopenia, including fatal cases.
These reactions are immunological in nature. They can occur even if the drug has been taken many times before without any complications.
Typical symptoms of agranulocytosis are lesions of the mucous membranes (oral cavity and pharynx, anorectal region and genital organs), sore throat, and fever. It should be borne in mind that if the patient receives antibiotic therapy, then the typical manifestations of agranulocytosis may be minimally expressed. The erythrocyte sedimentation rate increases significantly, while lymph node enlargement is mild or absent.
Typical symptoms of thrombocytopenia are an increased tendency to bleeding and the appearance of petechiae on the skin and mucous membranes. In the event of the development of the above disorders in the blood and lymphatic system, it is necessary to stop taking the drug and conduct a detailed clinical blood test (see section “Special instructions”).
Interaction
Interaction
The simultaneous use of metamizole with other non-narcotic analgesics can lead to mutual enhancement of toxic effects.
Tricyclic antidepressants, oral contraceptives, and allopurinol disrupt the metabolism of metamizole in the liver and increase its toxicity.
Barbiturates, phenylbutazone and other inducers of microsomal liver enzymes weaken the effect of metamizole. Sedatives and tranquilizers enhance the analgesic effect of the drug. Concomitant use with chlorpromazine or other phenothiazine derivatives can lead to the development of severe hyperthermia.
Radiocontrast agents, colloidal blood substitutes and penicillin should not be used during treatment with metamizole.
Metamizole, displacing oral hypoglycemic drugs, indirect anticoagulants, glucocorticosteroids and indomethacin from protein binding, increases their activity.
Myelotoxic drugs enhance the hematotoxicity of the drug.
Overdose
Overdose
Symptoms
In case of overdose, the following symptoms may occur: nausea, vomiting, abdominal pain, decreased renal function/acute renal failure with oliguria (for example, due to the development of interstitial nephritis), more rarely, symptoms from the central nervous system (dizziness, drowsiness, tinnitus, delirium, impaired consciousness, coma, convulsions) and a sharp decrease in blood pressure (sometimes progressing to shock), as well as heart rhythm disturbances (tachycardia), hypothermia, shortness of breath, acute agranulocytosis, hemorrhagic syndrome, acute liver failure, respiratory muscle paralysis. After high doses, excretion of a non-toxic metabolite (rubazonic acid) through the kidneys may cause red urine.
Treatment
If no more than 1-2 hours have passed after taking the drug, you can induce vomiting and perform gastric lavage through a tube; give saline laxatives, activated carbon. In case of overdose, forced diuresis is indicated. The main metabolite (4-N-methylaminoantipyrine) can be eliminated by hemodialysis, hemofiltration, hemoperfusion or plasma filtration. With the development of convulsive syndrome, intravenous administration of diazepam and fast-acting barbiturates.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 8–25 °C
Shelf life
Shelf life
4 years.
Do not use the drug after the expiration date indicated on the package.
Manufacturer
Manufacturer
Zentiva Private Limited, India
Additional information
| Shelf life | 4 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In a light-protected place, at 8-25 °C |
| Manufacturer | Zentiva Private Limited, India |
| Medication form | pills |
| Brand | Zentiva Private Limited |
Other forms…
Related products
Buy Baralgin M, tablets 500 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.